Volume 13, Issue 3 (Summer 2025)
Iran J Health Sci 2025, 13(3): 183-194 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Addissouky T A. Integrating Bioartificial Liver Systems and Liver Organoids for Enhancing Tertiary Prevention of Liver Diseases: A Review. Iran J Health Sci 2025; 13 (3) :183-194
URL: http://jhs.mazums.ac.ir/article-1-1015-en.html
URL: http://jhs.mazums.ac.ir/article-1-1015-en.html
New burg El-Arab Hospital, Ministry of Health, Alexandria, Egypt. , tedesoky@gmail.com
Full-Text [PDF 1062 kb]
(730 Downloads)
| Abstract (HTML) (1232 Views)
Full-Text: (271 Views)
Introduction
The liver is a vital organ responsible for a wide range of essential metabolic processes, including detoxification, protein synthesis, and the production of biochemicals necessary for digestion. It plays a central role in maintaining homeostasis by regulating blood sugar levels, lipid metabolism, and detoxifying harmful substances. Additionally, the liver is crucial for the synthesis of bile, which aids in digestion and facilitates fat metabolism [1-3]. The regenerative capacity of the liver is remarkable. However, severe damage can overwhelm this potential, leading to chronic liver diseases such as cirrhosis, hepatitis, and liver cancer. These conditions represent a significant global health burden, with liver diseases being a leading cause of morbidity and mortality worldwide [4-9].
Despite advances in medical research, liver diseases remain challenging to treat, primarily due to the complex and multifaceted functions of the liver. Conventional treatments, such as liver transplantation, are limited by the availability of donor organs, high costs, and the risk of immune rejection [10-13]. Moreover, drug development for liver diseases is particularly difficult, as the liver’s unique metabolic functions can cause unpredictable drug interactions and toxicities. Many potential drugs fail in clinical trials due to unforeseen liver toxicity, highlighting the critical need for reliable preclinical liver models to predict human responses more accurately. Current in vitro and animal models often fail to mimic the complexity of human liver biology, leading to a high rate of failure in drug development pipelines [14-16].
Given the limitations of existing treatment options and the high failure rate of drugs targeting liver diseases, there is an urgent need for advanced liver-mimicking systems. These systems should accurately replicate the structural and functional properties of the human liver to improve disease modeling, drug screening, and therapeutic development. Bioartificial liver (BAL) support systems, liver organoids, liver-on-a-chip models, and 3D bio-printed liver tissues are emerging technologies that hold tremendous potential to bridge the gap between preclinical models and clinical realities. These novel approaches aim to offer more physiologically relevant platforms for studying liver function, disease progression, and drug metabolism, thereby accelerating the development of new treatments for liver diseases [17, 18]. In this context, a critical question arises: How can liver organoids enhance the efficacy and clinical applicability of BAL support systems in the treatment and modeling of liver diseases? This study reviews current advances and challenges in both technologies, aiming to identify knowledge gaps and propose future directions for integrating these approaches to improve liver disease research and therapy.
Materials and Methods
This narrative review comprehensively evaluates recent advances in BAL support systems and liver organoid technologies relevant to liver disease modeling and therapeutic development. The PICO framework was used to form the research question: Human-relevant liver models (population), BAL systems and liver organoids (intervention), conventional models or comparative technologies (comparison), and outcomes related to metabolic functionality and drug screening efficacy (outcome). The search strategy targeted articles published from January 2015 to May 2024. Four major electronic databases were searched: PubMed, Scopus, Web of Science (WoS), and Embase. The search terms combined controlled vocabulary (MeSH terms in PubMed) and free-text keywords related to liver regenerative technologies, including but not limited to: “bioartificial liver”, “liver organoids”, “drug screening”, “liver disease modeling”, “stem cell-derived hepatocytes”, “3D bioprinting”, “liver-on-a-chip”, and “artificial intelligence in liver modeling”. Boolean operators (AND, OR) were used to refine and expand the search, ensuring comprehensive coverage of relevant literature. Table 1 outlines the study selection process.
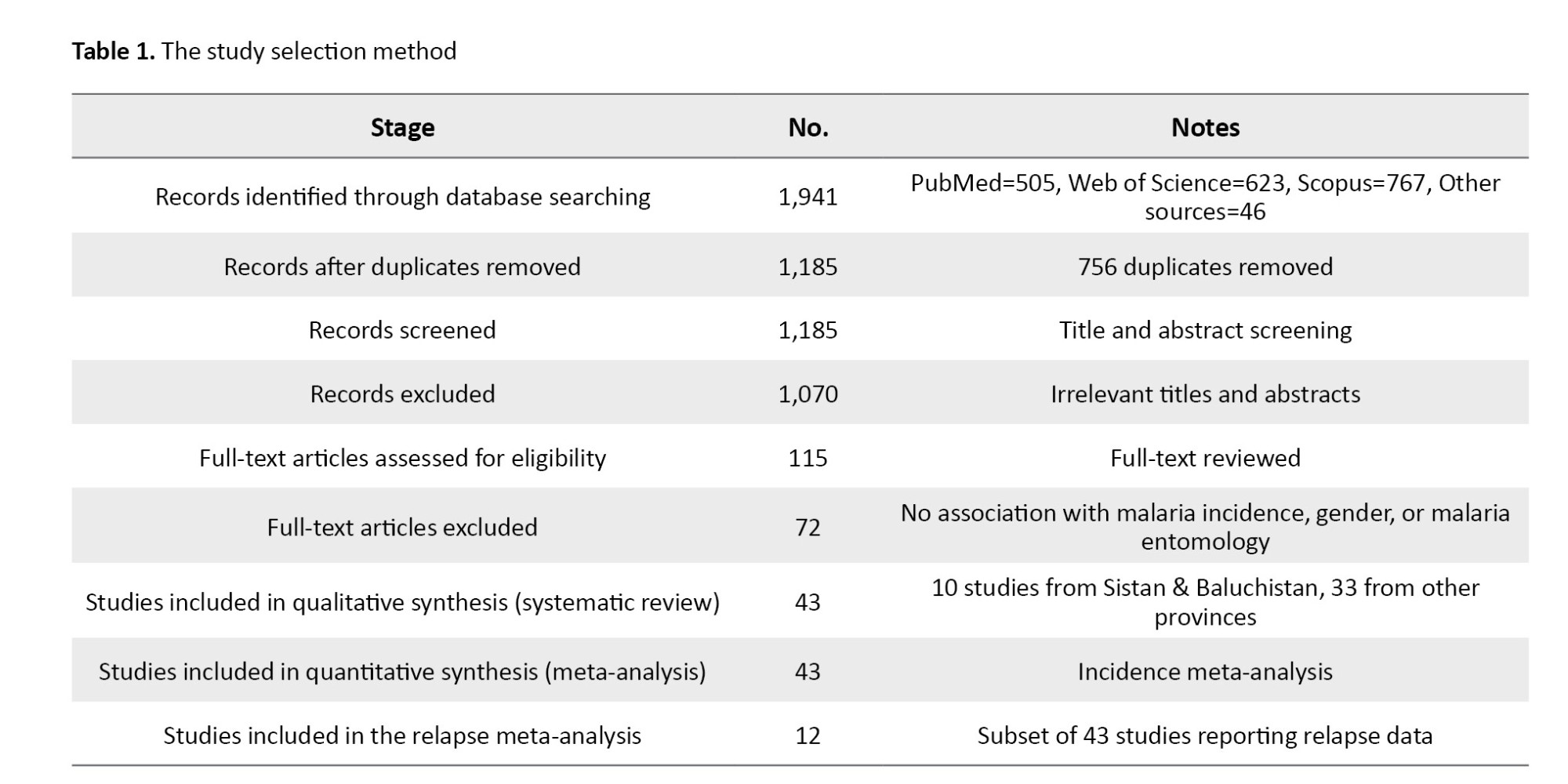
Included studies were: Original research articles, clinical trials, and comprehensive reviews focusing on BAL systems and liver organoids applied to liver disease modeling, drug screening, or regenerative therapeutics; studies involving human cells, stem cell-derived models, or clinically relevant animal models; and articles published in English within the specified timeframe. Excluded studies were: Conference abstracts, editorials, commentaries, and grey literature lacking full peer review, studies not directly related to BAL or liver organoid technologies, and non-English articles. Duplicates were identified and removed using EndNote software, version X9. Two independent reviewers screened titles and abstracts for relevance. Full-text articles were retrieved for potentially eligible studies and reviewed in detail. Any disagreement between reviewers was resolved by discussion or consultation with a third reviewer.
The extracted data were study design, cell sources, bioreactor configurations, extracellular matrix (ECM) innovations, clinical trial outcomes, drug screening applications, and integration of emerging technologies such as AI and 3D bioprinting. To ensure validity of data, only peer-reviewed studies were included, and cross-referencing of bibliographies was performed to identify additional relevant articles. The use of multiple databases and independent screening minimized selection bias. The review adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for narrative reviews to enhance transparency and reproducibility.
Results
BAL support systems
Overview and principles
The BAL systems are designed to provide temporary hepatic support in patients with acute liver failure by replicating essential liver functions. These hybrid devices combine biological components such as functional hepatocytes with mechanical systems to perform detoxification, metabolic support, and biosynthesis. Typically, BAL systems consist of a bioreactor containing viable liver cells—sourced from human or animal origins—and an extracorporeal circulation system facilitating toxin removal and metabolic exchange. The primary aim is to sustain liver function until endogenous regeneration or organ transplantation is feasible [19-24].
Recent advances
A significant challenge in BAL system development is to find sources and maintain robust, functional hepatocytes that closely mimic human liver physiology. Recent advances have focused on optimizing primary human hepatocytes, induced pluripotent stem cell (iPSC)-derived hepatocytes, and genetically engineered animal-derived cells. The iPSC-derived hepatocytes are promising due to their proliferative capacity and differentiation potential, offering renewable and scalable cell sources for BAL applications [25, 26].
Innovations in bioreactor technology have enhanced oxygen and nutrient transfer, thereby improving cell viability and function during extended operation. Hollow-fiber bioreactors, wherein hepatocytes are cultured within semipermeable fibers, have undergone refinements optimizing nutrient exchange and waste removal. Perfusion-based systems further emulate the liver’s dynamic microenvironment, supporting improved cellular longevity and function [27-30].
The ECM plays a critical role in maintaining hepatocyte phenotype and promoting tissue regeneration. Advances in ECM scaffolds—including decellularized liver matrices and synthetic hydrogels—offer biochemical and structural support that enhances hepatocyte adhesion, differentiation, and survival within BAL devices [31, 32].
Clinical applications and trials
BAL systems such as HepaLife and ELAD have been evaluated in clinical trials as bridging therapies for acute liver failure and transplantation candidates. Early-phase trials demonstrated transient improvement in liver function and patient survival. However, long-term efficacy and safety in larger randomized cohorts remain under investigation. Current research focuses on optimizing cell sources, bioreactor designs, and ECM scaffolds to enhance clinical outcomes [33-35].
Challenges and future directions
Despite progress, challenges include sustained functional cell sources, improved bioreactor microenvironment control, and physiologically relevant ECM integration. Risks of immune rejection and zoonotic infections, especially with animal-derived cells, pose additional hurdles. Future efforts should emphasize human-derived or genetically modified cells, advanced bioreactor technology, and novel biomaterials. Integration of emerging approaches such as 3D bioprinting and organ-on-a-chip platforms could further improve BAL functionality and facilitate clinical translation [36].
Liver organoids technology
Definition and characteristics
Liver organoids are three-dimensional (3D) self-organizing structures derived from stem cells or adult liver tissue that recapitulate key aspects of liver architecture and function (Table 2). They comprise hepatocytes, cholangiocytes, and other non-parenchymal cells, providing advanced platforms for liver development, disease modeling, and drug screening in vitro [37]. Artificial intelligence (AI) technologies are increasingly applied to optimize BAL bioreactor performance by monitoring parameters such as oxygenation, nutrient supply, pH, and metabolic activity in real time. AI-driven systems enable dynamic operational adjustments, improving hepatocyte viability and function while reducing human error. These predictive models also facilitate early detection of system failures, promoting more robust bioreactor operation [21].
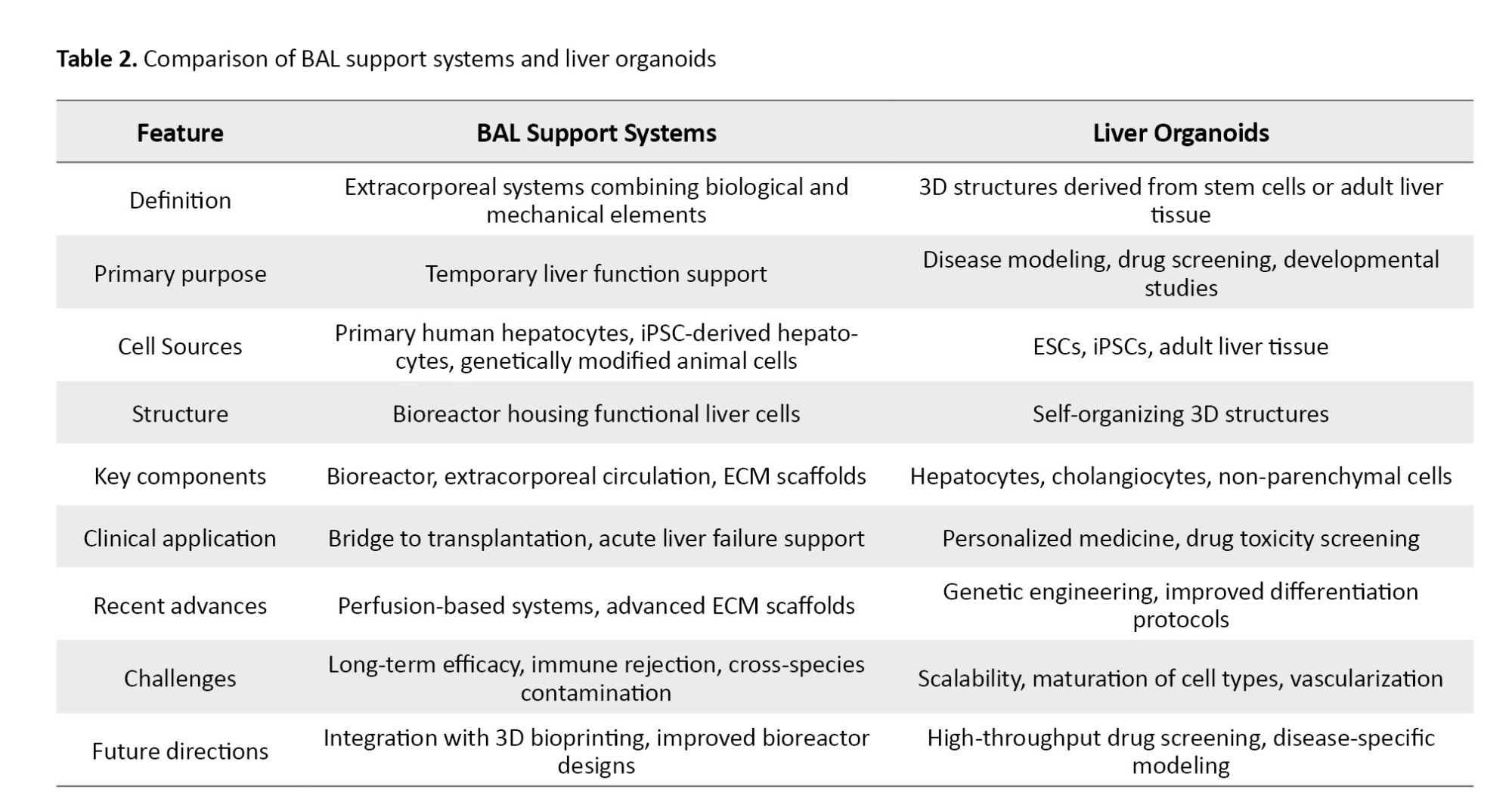
Recent developments
Embryonic stem cells (ESCs) and iPSCs can be differentiated into hepatocyte-like cells that self-assemble into 3D liver organoids. Advances in differentiation protocols and culture conditions have enhanced organoid efficiency and metabolic function, enabling applications in disease modeling and drug screening, including albumin production and bile acid metabolism [19].
Adult liver-derived organoids, generated from progenitor and hepatic stellate cells isolated from liver biopsies, retain physiological characteristics of native tissue. Optimized culture conditions have improved their long-term stability and functional capacity, supporting regenerative medicine and personalized therapeutic approaches [30].
CRISPR-Cas9 and other gene editing technologies have been employed to introduce or correct mutations in liver organoids, facilitating studies of genetic liver diseases such as non-alcoholic fatty liver disease, hepatitis, and liver cancer. These engineered models enable personalized disease modeling and targeted drug testing [33]. For more information regarding recent developments in liver-mimicking technologies (Table 3).
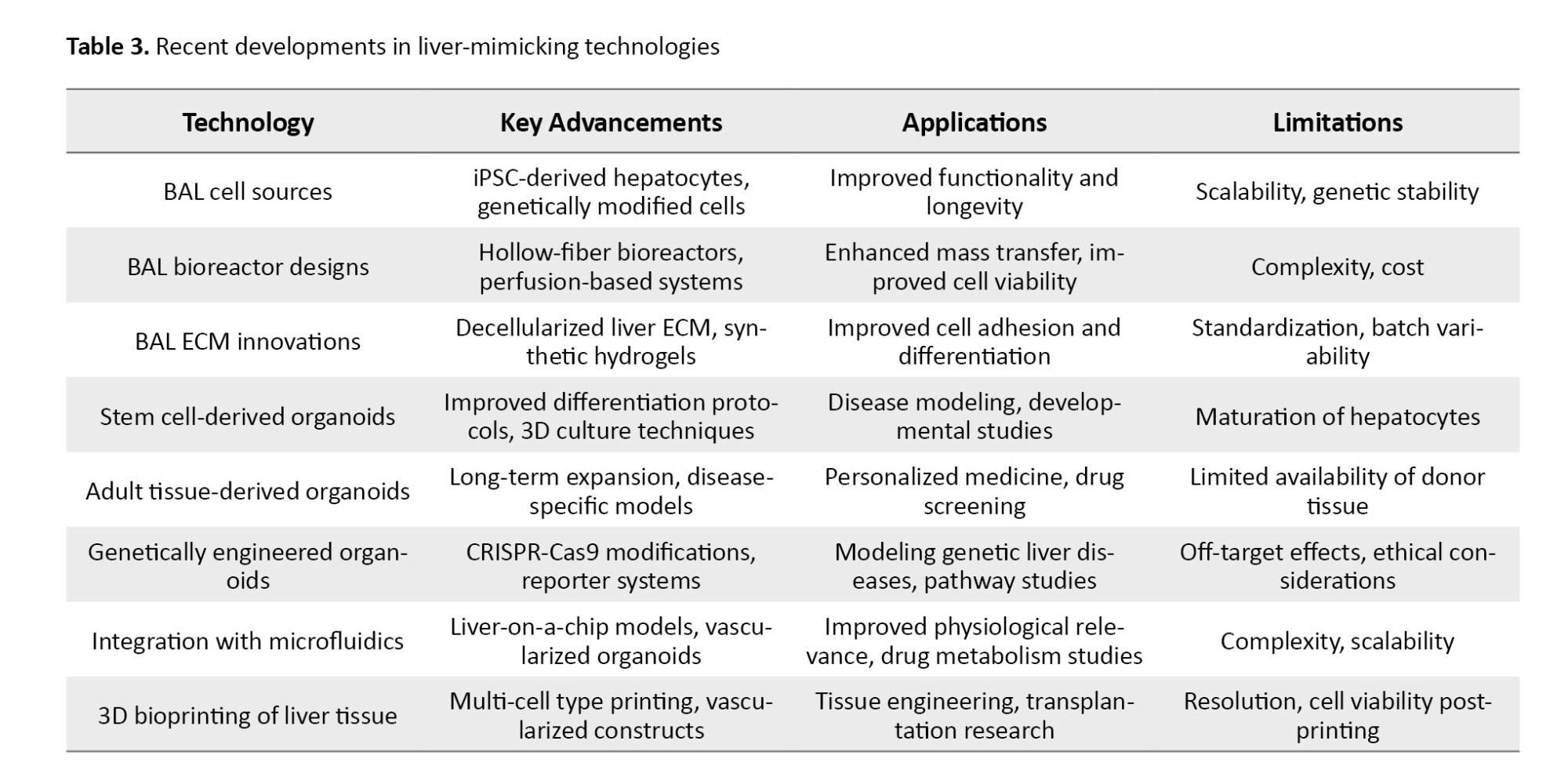
Synergistic integration of emerging technologies
Combining BAL systems, liver organoids, liver-on-a-chip models, 3D bioprinting, and AI holds promise for creating robust, scalable, and clinically relevant liver models. For example, 3D bioprinting can provide precise ECM scaffolds, enhancing organoid maturation. Liver-on-a-chip platforms simulate physiological conditions to improve functionality and drug response prediction. AI can optimize bioreactor performance in real time. Challenges remain in hepatocyte maturation, functional alignment with primary cells, and scalability, which must be addressed to advance clinical translation [34].
Applications in disease modeling
Liver organoids have been widely used as models for studying liver diseases, including viral hepatitis, liver fibrosis, and liver cancer. These organoids can replicate the pathophysiological processes of liver diseases in a controlled environment, allowing researchers to investigate disease mechanisms and identify potential therapeutic targets. For example, liver organoids have been used to model the progression of hepatitis B and C infections, as well as to study the fibrotic response to chronic liver injury. The ability to generate patient-specific organoids from iPSCs or adult tissue biopsies has opened up new possibilities for personalized medicine and precision therapies [35].
Organoid-based drug screening platforms
Liver organoids offer a promising platform for high-throughput drug screening due to their ability to replicate liver function and disease processes in vitro. These organoids can be used to test the efficacy and toxicity of new drugs in a physiologically relevant setting, improving the predictive power of preclinical studies. Recent advancements in automation and miniaturization have enabled the development of organoid-based drug screening platforms that can process large numbers of compounds simultaneously. This approach has the potential to accelerate the discovery of new treatments for liver diseases by providing more reliable data on drug efficacy and safety [9].
Advanced liver-on-a-chip models
Liver-on-a-chip models utilize microfluidic technologies to recreate the complex microenvironment of the liver in vitro. These systems integrate liver cells into microfluidic devices that simulate the flow of blood and nutrients, enabling researchers to study liver function in a more physiologically relevant setting. The microfluidic channels in liver-on-a-chip devices can be designed to mimic the architecture of the liver’s vasculature, allowing for precise control over nutrient delivery, waste removal, and oxygenation. This technology provides a powerful platform for studying liver function, disease progression, and drug metabolism [16, 18, 25, 26].
Recent innovations
Recent advancements in microfluidic technology have led to the development of multi-organ-on-a-chip systems, which integrate liver-on-a-chip devices with other organ models, such as heart, kidney, or lung chips. These systems allow researchers to study the interactions between different organs and how these interactions influence liver function and drug metabolism. Multi-organ-on-a-chip models have the potential to provide a more comprehensive understanding of drug toxicity and efficacy by replicating the complex interplay between organs in the human body [27].
Perfusion-based liver-on-a-chip systems have been developed to more closely mimic the dynamic environment of the liver. These systems use continuous perfusion to supply liver cells with nutrients and oxygen while removing waste products, replicating the conditions of the liver’s blood flow. Perfusion-based systems have been shown to improve the longevity and functionality of liver cells in vitro, making them more suitable for long-term studies of liver function and disease [28-32].
The integration of biosensors into liver-on-a-chip devices has enabled real-time monitoring of liver cell activity and function. These biosensors can detect key biomarkers of liver function, such as glucose, lactate, and albumin, as well as indicators of liver injury, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The ability to monitor liver cell function in real-time provides valuable insights into the effects of drugs and toxins on liver health, allowing researchers to identify potential liver toxicities at an early stage [33-35].
Applications in drug toxicity testing and metabolism studies
Liver-on-a-chip models are widely used in drug toxicity testing and metabolism studies due to their ability to replicate human liver function more accurately than traditional in vitro models. These systems can be used to assess the hepatotoxicity of new drugs and to study how drugs are metabolized in the liver. By providing a more physiologically relevant environment for liver cells, liver-on-a-chip models can improve the predictive power of preclinical studies and reduce the likelihood of DILI in clinical trials [9, 16, 18].
3D bioprinting of liver tissue
Principles for liver tissue engineering
3D bioprinting is an advanced technology that enables the precise fabrication of liver tissue constructs by layering bioinks containing liver cells and biomaterials. This technique allows for the creation of complex 3D structures that closely mimic the architecture and function of the liver. The ability to control the spatial arrangement of cells and ECM components in 3D bioprinted liver tissues provides a powerful tool for tissue engineering and regenerative medicine [25, 26].
Recent advances
Recent advancements in bioinks and 3D bioprinting techniques have significantly improved the quality and functionality of bioprinted liver tissues. Bioinks composed of natural ECM components, such as collagen, laminin, and fibrin, have been developed to provide a more physiologically relevant environment for liver cells. Additionally, advances in bioprinting technologies, such as multi-material printing and high-resolution printing, have enabled the creation of more complex and functional liver tissue constructs. These advancements have brought 3D bioprinted liver tissues closer to clinical applications in regenerative medicine and drug testing [27].
Applications in regenerative medicine and drug testing
3D bioprinted liver tissues have shown great potential for use in regenerative medicine, particularly for treating liver diseases and injuries. These tissues can be used to replace damaged liver tissue or to support liver regeneration in patients with liver failure. In addition to their therapeutic applications, 3D bioprinted liver tissues are being used as models for drug testing and toxicity screening. These tissues provide a more accurate representation of human liver function than traditional 2D cell cultures, making them a valuable tool for preclinical drug development [28].
Challenges and future prospects
Despite the significant progress made in 3D bioprinting of liver tissue, several challenges remain. These include the need for more advanced bioinks that can support long-term liver function, as well as the development of bioprinting techniques that can recreate the full complexity of liver architecture. Additionally, the scalability of 3D bioprinting technologies for large-scale production of functional liver tissues remains a major obstacle. Future research should focus on addressing these challenges by developing more advanced bioinks, improving bioprinting technologies, and optimizing the scalability of bioprinted liver tissues for clinical applications [29].
AI and machine learning technologies in liver modeling
AI and machine learning technologies are increasingly being used to design more accurate and predictive liver models. AI-driven algorithms can analyze large datasets from liver cells, organoids, and liver-on-a-chip systems to identify key factors that influence liver function and disease progression. These insights can be used to optimize the design of liver models, improving their accuracy and relevance for drug testing and disease modeling. AI can also be used to predict how liver cells will respond to different environmental conditions or drug treatments, enabling the development of more personalized liver models [30].
Machine learning algorithms have shown great promise in predicting drug-induced liver injury (DILI), one of the leading causes of drug failure in clinical trials. By analyzing large datasets from preclinical studies, clinical trials, and electronic health records, machine learning models can identify patterns associated with DILI and predict which drugs are likely to cause liver injury. These predictive models can significantly reduce the risk of DILI in drug development by identifying potentially hepatotoxic compounds early in the drug discovery process [31].
The integration of AI with experimental liver models, such as liver organoids and liver-on-a-chip systems, has the potential to revolutionize liver research. AI-driven analyses can provide real-time insights into liver cell function and disease progression, enabling researchers to optimize experimental conditions and identify potential therapeutic targets more efficiently. Additionally, AI can be used to analyze data from liver models in a more comprehensive and systematic manner, improving the accuracy and reliability of preclinical studies [32].
Emerging technologies and future directions
Single-cell sequencing in liver model development
Single-cell sequencing technologies are providing new insights into the cellular heterogeneity of the liver and how different cell types contribute to liver function and disease. These technologies allow researchers to analyze the gene expression profiles of individual liver cells, providing a more detailed understanding of liver biology at the single-cell level. This information can be used to develop more accurate liver models that replicate the diversity of cell types present in the liver, improving the relevance of these models for studying liver disease and drug toxicity [33].
CRISPR-Cas9 gene editing for personalized liver models
CRISPR-Cas9 gene editing technology is being used to develop personalized liver models by introducing patient-specific mutations or correcting genetic defects in liver cells, as depicted in Figure 1. By generating liver organoids or liver-on-a-chip models with patient-specific genetic backgrounds, researchers can study how these mutations affect liver function and disease progression, as well as test potential therapies in a more personalized manner. CRISPR-Cas9 technology also has the potential to be used in combination with other emerging technologies, such as 3D bioprinting and AI-driven liver models, to create more accurate and personalized liver models for clinical applications [34, 35].
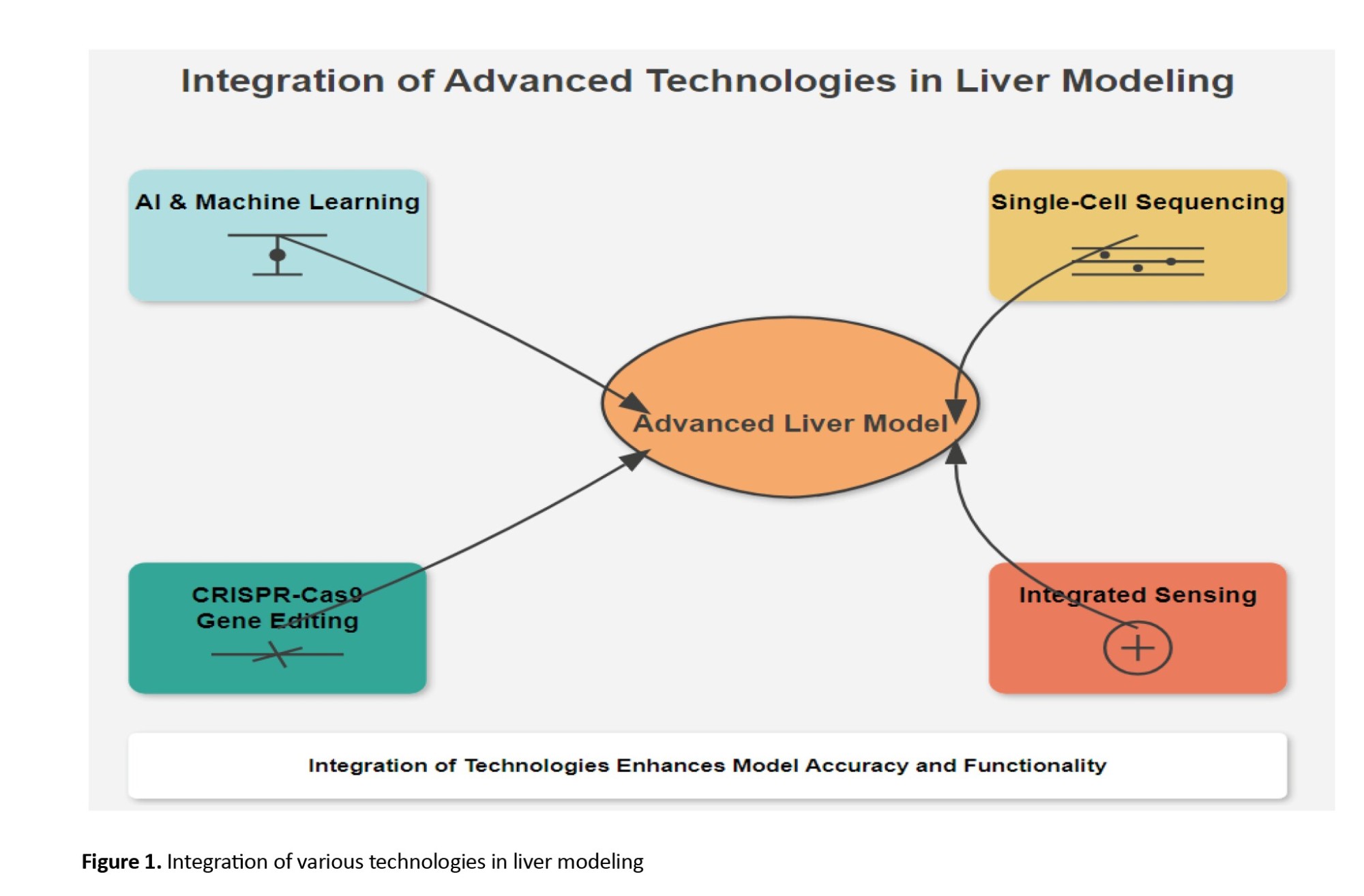
Organ-on-a-chip systems with integrated sensing capabilities
The integration of biosensors and microfluidic technologies into organ-on-a-chip systems is an exciting area of research that has the potential to revolutionize liver modeling. These advanced systems can provide real-time monitoring of liver cell function and metabolic activity, enabling researchers to study the effects of drugs and toxins on liver health in a more dynamic and physiologically relevant manner. The combination of biosensors with liver-on-a-chip models could also improve the accuracy of drug toxicity testing and accelerate the development of new therapies for liver diseases [9].
Combining organoids with BAL systems for enhanced functionality
The combination of liver organoids with BAL systems represents a promising approach for enhancing the functionality of both technologies. Organoids can provide a more physiologically relevant source of liver cells for BAL systems, while BAL systems can provide the necessary support for organoids to function more effectively in a clinical setting. This combination could lead to the development of more advanced liver support systems that can better mimic human liver function and provide more effective treatments for patients with liver failure [16].
Clinical translation and commercialization of advanced liver models
Several advanced liver models, such as liver-on-a-chip systems and liver organoids, have shown promising results in preclinical studies and are beginning to be translated into clinical applications. These models are being used to test new drugs, study liver diseases, and develop personalized treatments for patients with liver disorders. However, the clinical translation of these technologies is still in its early stages, and more research is needed to demonstrate their efficacy and safety in larger patient populations [18, 25-27].
One of the major challenges in the clinical translation of advanced liver models is the scalability of these technologies. Many of the current liver-on-a-chip systems, organoids, and 3D bioprinted liver tissues are difficult to produce on a large scale, limiting their clinical applicability. Additionally, the manufacturing processes for these models are often complex and expensive, making it challenging to produce them in a cost-effective manner. Future research should focus on developing more scalable and cost-effective manufacturing processes for advanced liver models to facilitate their clinical translation [28-30].
The market potential for advanced liver models is significant, particularly in the pharmaceutical and biotechnology industries. These models have the potential to revolutionize drug development by providing more accurate [38].
Discussion
The development and integration of advanced liver regenerative technologies, such as BAL support systems, liver organoids, liver-on-a-chip platforms, 3D bioprinting, and AI, represent a transformative frontier in hepatology and translational medicine. Liver diseases, ranging from acute liver failure to chronic conditions like cirrhosis and hepatocellular carcinoma, impose significant morbidity, mortality, and economic burden worldwide. Conventional treatment modalities, including liver transplantation, remain constrained by donor shortages, immune complications, and high costs. Therefore, innovative alternatives that can replicate liver function ex vivo or model disease in vitro are critically needed to improve patient outcomes and accelerate drug development [38, 39]. This review underscores the clinical and biomedical relevance of these emerging platforms.
BAL systems provide crucial bridge therapy for patients awaiting transplantation, yet their efficacy is contingent upon optimizing cell sources, bioreactor designs, and ECM components. Previous studies have noted similar challenges in achieving consistent functionality in BAL devices, reinforcing the need for ongoing optimization (consistent with earlier findings) [40, 41]. Liver organoids, derived from stem cells, offer scalable, patient-specific models that recapitulate key aspects of liver physiology and pathology, facilitating personalized medicine and mechanistic studies. However, this aligns with earlier research highlighting the limitations of organoid models in fully mimicking adult hepatocyte functionality (contrary to earlier studies). Liver-on-a-chip devices combine microfluidics with organoids or primary cells to recreate the liver microenvironment under dynamic conditions, enhancing the physiological relevance of in vitro models. The advent of 3D bioprinting allows for precise spatial organization of multiple liver cell types within biomimetic scaffolds, promising improved tissue architecture and function. AI technologies augment these platforms by enabling real-time monitoring, predictive modeling, and automation, addressing limitations in reproducibility and scalability [42, 43].
Despite these advances, several challenges require attention. Achieving full adult hepatocyte functionality in stem cell-derived organoids remains elusive, limiting their predictive power. This complexity mirrors previous findings that emphasize the difficulties in replicating the intricate multicellular interactions and zonation of liver physiology in vitro or in BAL devices (consistent with earlier studies). Scaling up for clinical applications faces hurdles related to cost, manufacturing consistency, and regulatory approval. Additionally, the risk of immune rejection and zoonotic contamination in BAL systems using animal cells must be carefully managed. Addressing these challenges necessitates a multidisciplinary effort involving cell biology, bioengineering, computational sciences, and clinical expertise [44].
Conclusion
This review study underscores major progress in liver regenerative medicine and bioengineering, highlighting technologies such as BAL systems, liver organoids, liver-on-a-chip platforms, 3D bioprinting, and AI. Key challenges remain, including fully maturing hepatocytes in organoids, replicating liver microenvironments, scalability, and clinical application. Combining these technologies offers promising solutions for liver modeling, drug screening, and alternatives to liver transplantation. Future research should focus on improving cell maturity, AI-enhanced bioreactor designs, and scalable culture methods. To advance clinical use, a multidisciplinary effort is needed, combining stem cell biology, tissue engineering, microfluidics, and computational modeling. Standardizing organoid protocols can improve reproducibility. Prioritizing AI-driven bioreactor systems can enhance cell function during culture. Collaboration among academic and industry settings and regulators is essential for setting quality and safety standards. The use of patient-derived cells can support personalized medicine. Expanding preclinical studies to large animal studies and conducting well-designed clinical trials are critical to validating these technologies for real-world applications.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The author thanks all the researchers who have made great efforts in their studies. Moreover, we are grateful to this journal's editors, reviewers, and readers.
References
The liver is a vital organ responsible for a wide range of essential metabolic processes, including detoxification, protein synthesis, and the production of biochemicals necessary for digestion. It plays a central role in maintaining homeostasis by regulating blood sugar levels, lipid metabolism, and detoxifying harmful substances. Additionally, the liver is crucial for the synthesis of bile, which aids in digestion and facilitates fat metabolism [1-3]. The regenerative capacity of the liver is remarkable. However, severe damage can overwhelm this potential, leading to chronic liver diseases such as cirrhosis, hepatitis, and liver cancer. These conditions represent a significant global health burden, with liver diseases being a leading cause of morbidity and mortality worldwide [4-9].
Despite advances in medical research, liver diseases remain challenging to treat, primarily due to the complex and multifaceted functions of the liver. Conventional treatments, such as liver transplantation, are limited by the availability of donor organs, high costs, and the risk of immune rejection [10-13]. Moreover, drug development for liver diseases is particularly difficult, as the liver’s unique metabolic functions can cause unpredictable drug interactions and toxicities. Many potential drugs fail in clinical trials due to unforeseen liver toxicity, highlighting the critical need for reliable preclinical liver models to predict human responses more accurately. Current in vitro and animal models often fail to mimic the complexity of human liver biology, leading to a high rate of failure in drug development pipelines [14-16].
Given the limitations of existing treatment options and the high failure rate of drugs targeting liver diseases, there is an urgent need for advanced liver-mimicking systems. These systems should accurately replicate the structural and functional properties of the human liver to improve disease modeling, drug screening, and therapeutic development. Bioartificial liver (BAL) support systems, liver organoids, liver-on-a-chip models, and 3D bio-printed liver tissues are emerging technologies that hold tremendous potential to bridge the gap between preclinical models and clinical realities. These novel approaches aim to offer more physiologically relevant platforms for studying liver function, disease progression, and drug metabolism, thereby accelerating the development of new treatments for liver diseases [17, 18]. In this context, a critical question arises: How can liver organoids enhance the efficacy and clinical applicability of BAL support systems in the treatment and modeling of liver diseases? This study reviews current advances and challenges in both technologies, aiming to identify knowledge gaps and propose future directions for integrating these approaches to improve liver disease research and therapy.
Materials and Methods
This narrative review comprehensively evaluates recent advances in BAL support systems and liver organoid technologies relevant to liver disease modeling and therapeutic development. The PICO framework was used to form the research question: Human-relevant liver models (population), BAL systems and liver organoids (intervention), conventional models or comparative technologies (comparison), and outcomes related to metabolic functionality and drug screening efficacy (outcome). The search strategy targeted articles published from January 2015 to May 2024. Four major electronic databases were searched: PubMed, Scopus, Web of Science (WoS), and Embase. The search terms combined controlled vocabulary (MeSH terms in PubMed) and free-text keywords related to liver regenerative technologies, including but not limited to: “bioartificial liver”, “liver organoids”, “drug screening”, “liver disease modeling”, “stem cell-derived hepatocytes”, “3D bioprinting”, “liver-on-a-chip”, and “artificial intelligence in liver modeling”. Boolean operators (AND, OR) were used to refine and expand the search, ensuring comprehensive coverage of relevant literature. Table 1 outlines the study selection process.

Included studies were: Original research articles, clinical trials, and comprehensive reviews focusing on BAL systems and liver organoids applied to liver disease modeling, drug screening, or regenerative therapeutics; studies involving human cells, stem cell-derived models, or clinically relevant animal models; and articles published in English within the specified timeframe. Excluded studies were: Conference abstracts, editorials, commentaries, and grey literature lacking full peer review, studies not directly related to BAL or liver organoid technologies, and non-English articles. Duplicates were identified and removed using EndNote software, version X9. Two independent reviewers screened titles and abstracts for relevance. Full-text articles were retrieved for potentially eligible studies and reviewed in detail. Any disagreement between reviewers was resolved by discussion or consultation with a third reviewer.
The extracted data were study design, cell sources, bioreactor configurations, extracellular matrix (ECM) innovations, clinical trial outcomes, drug screening applications, and integration of emerging technologies such as AI and 3D bioprinting. To ensure validity of data, only peer-reviewed studies were included, and cross-referencing of bibliographies was performed to identify additional relevant articles. The use of multiple databases and independent screening minimized selection bias. The review adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for narrative reviews to enhance transparency and reproducibility.
Results
BAL support systems
Overview and principles
The BAL systems are designed to provide temporary hepatic support in patients with acute liver failure by replicating essential liver functions. These hybrid devices combine biological components such as functional hepatocytes with mechanical systems to perform detoxification, metabolic support, and biosynthesis. Typically, BAL systems consist of a bioreactor containing viable liver cells—sourced from human or animal origins—and an extracorporeal circulation system facilitating toxin removal and metabolic exchange. The primary aim is to sustain liver function until endogenous regeneration or organ transplantation is feasible [19-24].
Recent advances
A significant challenge in BAL system development is to find sources and maintain robust, functional hepatocytes that closely mimic human liver physiology. Recent advances have focused on optimizing primary human hepatocytes, induced pluripotent stem cell (iPSC)-derived hepatocytes, and genetically engineered animal-derived cells. The iPSC-derived hepatocytes are promising due to their proliferative capacity and differentiation potential, offering renewable and scalable cell sources for BAL applications [25, 26].
Innovations in bioreactor technology have enhanced oxygen and nutrient transfer, thereby improving cell viability and function during extended operation. Hollow-fiber bioreactors, wherein hepatocytes are cultured within semipermeable fibers, have undergone refinements optimizing nutrient exchange and waste removal. Perfusion-based systems further emulate the liver’s dynamic microenvironment, supporting improved cellular longevity and function [27-30].
The ECM plays a critical role in maintaining hepatocyte phenotype and promoting tissue regeneration. Advances in ECM scaffolds—including decellularized liver matrices and synthetic hydrogels—offer biochemical and structural support that enhances hepatocyte adhesion, differentiation, and survival within BAL devices [31, 32].
Clinical applications and trials
BAL systems such as HepaLife and ELAD have been evaluated in clinical trials as bridging therapies for acute liver failure and transplantation candidates. Early-phase trials demonstrated transient improvement in liver function and patient survival. However, long-term efficacy and safety in larger randomized cohorts remain under investigation. Current research focuses on optimizing cell sources, bioreactor designs, and ECM scaffolds to enhance clinical outcomes [33-35].
Challenges and future directions
Despite progress, challenges include sustained functional cell sources, improved bioreactor microenvironment control, and physiologically relevant ECM integration. Risks of immune rejection and zoonotic infections, especially with animal-derived cells, pose additional hurdles. Future efforts should emphasize human-derived or genetically modified cells, advanced bioreactor technology, and novel biomaterials. Integration of emerging approaches such as 3D bioprinting and organ-on-a-chip platforms could further improve BAL functionality and facilitate clinical translation [36].
Liver organoids technology
Definition and characteristics
Liver organoids are three-dimensional (3D) self-organizing structures derived from stem cells or adult liver tissue that recapitulate key aspects of liver architecture and function (Table 2). They comprise hepatocytes, cholangiocytes, and other non-parenchymal cells, providing advanced platforms for liver development, disease modeling, and drug screening in vitro [37]. Artificial intelligence (AI) technologies are increasingly applied to optimize BAL bioreactor performance by monitoring parameters such as oxygenation, nutrient supply, pH, and metabolic activity in real time. AI-driven systems enable dynamic operational adjustments, improving hepatocyte viability and function while reducing human error. These predictive models also facilitate early detection of system failures, promoting more robust bioreactor operation [21].

Recent developments
Embryonic stem cells (ESCs) and iPSCs can be differentiated into hepatocyte-like cells that self-assemble into 3D liver organoids. Advances in differentiation protocols and culture conditions have enhanced organoid efficiency and metabolic function, enabling applications in disease modeling and drug screening, including albumin production and bile acid metabolism [19].
Adult liver-derived organoids, generated from progenitor and hepatic stellate cells isolated from liver biopsies, retain physiological characteristics of native tissue. Optimized culture conditions have improved their long-term stability and functional capacity, supporting regenerative medicine and personalized therapeutic approaches [30].
CRISPR-Cas9 and other gene editing technologies have been employed to introduce or correct mutations in liver organoids, facilitating studies of genetic liver diseases such as non-alcoholic fatty liver disease, hepatitis, and liver cancer. These engineered models enable personalized disease modeling and targeted drug testing [33]. For more information regarding recent developments in liver-mimicking technologies (Table 3).

Synergistic integration of emerging technologies
Combining BAL systems, liver organoids, liver-on-a-chip models, 3D bioprinting, and AI holds promise for creating robust, scalable, and clinically relevant liver models. For example, 3D bioprinting can provide precise ECM scaffolds, enhancing organoid maturation. Liver-on-a-chip platforms simulate physiological conditions to improve functionality and drug response prediction. AI can optimize bioreactor performance in real time. Challenges remain in hepatocyte maturation, functional alignment with primary cells, and scalability, which must be addressed to advance clinical translation [34].
Applications in disease modeling
Liver organoids have been widely used as models for studying liver diseases, including viral hepatitis, liver fibrosis, and liver cancer. These organoids can replicate the pathophysiological processes of liver diseases in a controlled environment, allowing researchers to investigate disease mechanisms and identify potential therapeutic targets. For example, liver organoids have been used to model the progression of hepatitis B and C infections, as well as to study the fibrotic response to chronic liver injury. The ability to generate patient-specific organoids from iPSCs or adult tissue biopsies has opened up new possibilities for personalized medicine and precision therapies [35].
Organoid-based drug screening platforms
Liver organoids offer a promising platform for high-throughput drug screening due to their ability to replicate liver function and disease processes in vitro. These organoids can be used to test the efficacy and toxicity of new drugs in a physiologically relevant setting, improving the predictive power of preclinical studies. Recent advancements in automation and miniaturization have enabled the development of organoid-based drug screening platforms that can process large numbers of compounds simultaneously. This approach has the potential to accelerate the discovery of new treatments for liver diseases by providing more reliable data on drug efficacy and safety [9].
Advanced liver-on-a-chip models
Liver-on-a-chip models utilize microfluidic technologies to recreate the complex microenvironment of the liver in vitro. These systems integrate liver cells into microfluidic devices that simulate the flow of blood and nutrients, enabling researchers to study liver function in a more physiologically relevant setting. The microfluidic channels in liver-on-a-chip devices can be designed to mimic the architecture of the liver’s vasculature, allowing for precise control over nutrient delivery, waste removal, and oxygenation. This technology provides a powerful platform for studying liver function, disease progression, and drug metabolism [16, 18, 25, 26].
Recent innovations
Recent advancements in microfluidic technology have led to the development of multi-organ-on-a-chip systems, which integrate liver-on-a-chip devices with other organ models, such as heart, kidney, or lung chips. These systems allow researchers to study the interactions between different organs and how these interactions influence liver function and drug metabolism. Multi-organ-on-a-chip models have the potential to provide a more comprehensive understanding of drug toxicity and efficacy by replicating the complex interplay between organs in the human body [27].
Perfusion-based liver-on-a-chip systems have been developed to more closely mimic the dynamic environment of the liver. These systems use continuous perfusion to supply liver cells with nutrients and oxygen while removing waste products, replicating the conditions of the liver’s blood flow. Perfusion-based systems have been shown to improve the longevity and functionality of liver cells in vitro, making them more suitable for long-term studies of liver function and disease [28-32].
The integration of biosensors into liver-on-a-chip devices has enabled real-time monitoring of liver cell activity and function. These biosensors can detect key biomarkers of liver function, such as glucose, lactate, and albumin, as well as indicators of liver injury, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The ability to monitor liver cell function in real-time provides valuable insights into the effects of drugs and toxins on liver health, allowing researchers to identify potential liver toxicities at an early stage [33-35].
Applications in drug toxicity testing and metabolism studies
Liver-on-a-chip models are widely used in drug toxicity testing and metabolism studies due to their ability to replicate human liver function more accurately than traditional in vitro models. These systems can be used to assess the hepatotoxicity of new drugs and to study how drugs are metabolized in the liver. By providing a more physiologically relevant environment for liver cells, liver-on-a-chip models can improve the predictive power of preclinical studies and reduce the likelihood of DILI in clinical trials [9, 16, 18].
3D bioprinting of liver tissue
Principles for liver tissue engineering
3D bioprinting is an advanced technology that enables the precise fabrication of liver tissue constructs by layering bioinks containing liver cells and biomaterials. This technique allows for the creation of complex 3D structures that closely mimic the architecture and function of the liver. The ability to control the spatial arrangement of cells and ECM components in 3D bioprinted liver tissues provides a powerful tool for tissue engineering and regenerative medicine [25, 26].
Recent advances
Recent advancements in bioinks and 3D bioprinting techniques have significantly improved the quality and functionality of bioprinted liver tissues. Bioinks composed of natural ECM components, such as collagen, laminin, and fibrin, have been developed to provide a more physiologically relevant environment for liver cells. Additionally, advances in bioprinting technologies, such as multi-material printing and high-resolution printing, have enabled the creation of more complex and functional liver tissue constructs. These advancements have brought 3D bioprinted liver tissues closer to clinical applications in regenerative medicine and drug testing [27].
Applications in regenerative medicine and drug testing
3D bioprinted liver tissues have shown great potential for use in regenerative medicine, particularly for treating liver diseases and injuries. These tissues can be used to replace damaged liver tissue or to support liver regeneration in patients with liver failure. In addition to their therapeutic applications, 3D bioprinted liver tissues are being used as models for drug testing and toxicity screening. These tissues provide a more accurate representation of human liver function than traditional 2D cell cultures, making them a valuable tool for preclinical drug development [28].
Challenges and future prospects
Despite the significant progress made in 3D bioprinting of liver tissue, several challenges remain. These include the need for more advanced bioinks that can support long-term liver function, as well as the development of bioprinting techniques that can recreate the full complexity of liver architecture. Additionally, the scalability of 3D bioprinting technologies for large-scale production of functional liver tissues remains a major obstacle. Future research should focus on addressing these challenges by developing more advanced bioinks, improving bioprinting technologies, and optimizing the scalability of bioprinted liver tissues for clinical applications [29].
AI and machine learning technologies in liver modeling
AI and machine learning technologies are increasingly being used to design more accurate and predictive liver models. AI-driven algorithms can analyze large datasets from liver cells, organoids, and liver-on-a-chip systems to identify key factors that influence liver function and disease progression. These insights can be used to optimize the design of liver models, improving their accuracy and relevance for drug testing and disease modeling. AI can also be used to predict how liver cells will respond to different environmental conditions or drug treatments, enabling the development of more personalized liver models [30].
Machine learning algorithms have shown great promise in predicting drug-induced liver injury (DILI), one of the leading causes of drug failure in clinical trials. By analyzing large datasets from preclinical studies, clinical trials, and electronic health records, machine learning models can identify patterns associated with DILI and predict which drugs are likely to cause liver injury. These predictive models can significantly reduce the risk of DILI in drug development by identifying potentially hepatotoxic compounds early in the drug discovery process [31].
The integration of AI with experimental liver models, such as liver organoids and liver-on-a-chip systems, has the potential to revolutionize liver research. AI-driven analyses can provide real-time insights into liver cell function and disease progression, enabling researchers to optimize experimental conditions and identify potential therapeutic targets more efficiently. Additionally, AI can be used to analyze data from liver models in a more comprehensive and systematic manner, improving the accuracy and reliability of preclinical studies [32].
Emerging technologies and future directions
Single-cell sequencing in liver model development
Single-cell sequencing technologies are providing new insights into the cellular heterogeneity of the liver and how different cell types contribute to liver function and disease. These technologies allow researchers to analyze the gene expression profiles of individual liver cells, providing a more detailed understanding of liver biology at the single-cell level. This information can be used to develop more accurate liver models that replicate the diversity of cell types present in the liver, improving the relevance of these models for studying liver disease and drug toxicity [33].
CRISPR-Cas9 gene editing for personalized liver models
CRISPR-Cas9 gene editing technology is being used to develop personalized liver models by introducing patient-specific mutations or correcting genetic defects in liver cells, as depicted in Figure 1. By generating liver organoids or liver-on-a-chip models with patient-specific genetic backgrounds, researchers can study how these mutations affect liver function and disease progression, as well as test potential therapies in a more personalized manner. CRISPR-Cas9 technology also has the potential to be used in combination with other emerging technologies, such as 3D bioprinting and AI-driven liver models, to create more accurate and personalized liver models for clinical applications [34, 35].

Organ-on-a-chip systems with integrated sensing capabilities
The integration of biosensors and microfluidic technologies into organ-on-a-chip systems is an exciting area of research that has the potential to revolutionize liver modeling. These advanced systems can provide real-time monitoring of liver cell function and metabolic activity, enabling researchers to study the effects of drugs and toxins on liver health in a more dynamic and physiologically relevant manner. The combination of biosensors with liver-on-a-chip models could also improve the accuracy of drug toxicity testing and accelerate the development of new therapies for liver diseases [9].
Combining organoids with BAL systems for enhanced functionality
The combination of liver organoids with BAL systems represents a promising approach for enhancing the functionality of both technologies. Organoids can provide a more physiologically relevant source of liver cells for BAL systems, while BAL systems can provide the necessary support for organoids to function more effectively in a clinical setting. This combination could lead to the development of more advanced liver support systems that can better mimic human liver function and provide more effective treatments for patients with liver failure [16].
Clinical translation and commercialization of advanced liver models
Several advanced liver models, such as liver-on-a-chip systems and liver organoids, have shown promising results in preclinical studies and are beginning to be translated into clinical applications. These models are being used to test new drugs, study liver diseases, and develop personalized treatments for patients with liver disorders. However, the clinical translation of these technologies is still in its early stages, and more research is needed to demonstrate their efficacy and safety in larger patient populations [18, 25-27].
One of the major challenges in the clinical translation of advanced liver models is the scalability of these technologies. Many of the current liver-on-a-chip systems, organoids, and 3D bioprinted liver tissues are difficult to produce on a large scale, limiting their clinical applicability. Additionally, the manufacturing processes for these models are often complex and expensive, making it challenging to produce them in a cost-effective manner. Future research should focus on developing more scalable and cost-effective manufacturing processes for advanced liver models to facilitate their clinical translation [28-30].
The market potential for advanced liver models is significant, particularly in the pharmaceutical and biotechnology industries. These models have the potential to revolutionize drug development by providing more accurate [38].
Discussion
The development and integration of advanced liver regenerative technologies, such as BAL support systems, liver organoids, liver-on-a-chip platforms, 3D bioprinting, and AI, represent a transformative frontier in hepatology and translational medicine. Liver diseases, ranging from acute liver failure to chronic conditions like cirrhosis and hepatocellular carcinoma, impose significant morbidity, mortality, and economic burden worldwide. Conventional treatment modalities, including liver transplantation, remain constrained by donor shortages, immune complications, and high costs. Therefore, innovative alternatives that can replicate liver function ex vivo or model disease in vitro are critically needed to improve patient outcomes and accelerate drug development [38, 39]. This review underscores the clinical and biomedical relevance of these emerging platforms.
BAL systems provide crucial bridge therapy for patients awaiting transplantation, yet their efficacy is contingent upon optimizing cell sources, bioreactor designs, and ECM components. Previous studies have noted similar challenges in achieving consistent functionality in BAL devices, reinforcing the need for ongoing optimization (consistent with earlier findings) [40, 41]. Liver organoids, derived from stem cells, offer scalable, patient-specific models that recapitulate key aspects of liver physiology and pathology, facilitating personalized medicine and mechanistic studies. However, this aligns with earlier research highlighting the limitations of organoid models in fully mimicking adult hepatocyte functionality (contrary to earlier studies). Liver-on-a-chip devices combine microfluidics with organoids or primary cells to recreate the liver microenvironment under dynamic conditions, enhancing the physiological relevance of in vitro models. The advent of 3D bioprinting allows for precise spatial organization of multiple liver cell types within biomimetic scaffolds, promising improved tissue architecture and function. AI technologies augment these platforms by enabling real-time monitoring, predictive modeling, and automation, addressing limitations in reproducibility and scalability [42, 43].
Despite these advances, several challenges require attention. Achieving full adult hepatocyte functionality in stem cell-derived organoids remains elusive, limiting their predictive power. This complexity mirrors previous findings that emphasize the difficulties in replicating the intricate multicellular interactions and zonation of liver physiology in vitro or in BAL devices (consistent with earlier studies). Scaling up for clinical applications faces hurdles related to cost, manufacturing consistency, and regulatory approval. Additionally, the risk of immune rejection and zoonotic contamination in BAL systems using animal cells must be carefully managed. Addressing these challenges necessitates a multidisciplinary effort involving cell biology, bioengineering, computational sciences, and clinical expertise [44].
Conclusion
This review study underscores major progress in liver regenerative medicine and bioengineering, highlighting technologies such as BAL systems, liver organoids, liver-on-a-chip platforms, 3D bioprinting, and AI. Key challenges remain, including fully maturing hepatocytes in organoids, replicating liver microenvironments, scalability, and clinical application. Combining these technologies offers promising solutions for liver modeling, drug screening, and alternatives to liver transplantation. Future research should focus on improving cell maturity, AI-enhanced bioreactor designs, and scalable culture methods. To advance clinical use, a multidisciplinary effort is needed, combining stem cell biology, tissue engineering, microfluidics, and computational modeling. Standardizing organoid protocols can improve reproducibility. Prioritizing AI-driven bioreactor systems can enhance cell function during culture. Collaboration among academic and industry settings and regulators is essential for setting quality and safety standards. The use of patient-derived cells can support personalized medicine. Expanding preclinical studies to large animal studies and conducting well-designed clinical trials are critical to validating these technologies for real-world applications.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The author thanks all the researchers who have made great efforts in their studies. Moreover, we are grateful to this journal's editors, reviewers, and readers.
References
- Ullah MI, Alameen AA, Al-Oanzi ZH, Eltayeb LB, Atif M, Munir MU, et al. Biological role of zinc in liver cirrhosis: An updated review. Biomedicines. 2023; 11(4):1094. [DOI:10.3390/biomedicines11041094] [PMID] [PMCID]
- Lubna S, Ahmad R. Clinical and biochemical understanding of Zinc interaction during liver diseases: A paradigm shift. Journal of Trace Elements in Medicine and Biology. 2023; 77:127130. [DOI:10.1016/j.jtemb.2023.127130] [PMID]
- Addissouky TA. Sepsis: A molecular odyssey from infection to organ failure. Journal of Cellular Immunology. 2025; 7(2):64-73. [DOI:10.33696/immunology.7.226]
- Li N, Zhao C, Zhang P, Wu S, Dou X, Xu S, et al. The role of gut microbiota associated metabolites in digestive disorders. Engineered Regeneration. 2024; 5(2):228-46. [DOI:10.1016/j.engreg.2024.04.003]
- Addissouky TA. Beyond insulin: Advancing frontiers in cell-based and genetic therapies for type 1 diabetes management. The Egyptian Journal of Internal Medicine. 2025; 37:84. [DOI:10.1186/s43162-025-00477-y]
- Liu R, Scimeca M, Sun Q, Melino G, Mauriello A, Shao C, et al. Harnessing metabolism of hepatic macrophages to aid liver regeneration. Cell Death & Disease. 2023; 14(8):1-10. [DOI:10.1038/s41419-023-06066-7] [PMID] [PMCID]
- Addissouky TA. Nanoscale frontiers in cancer diagnosis and therapy. Discover Chemistry. 2025; 2:159. [DOI:10.1007/s44371-025-00237-5]
- Montano-Loza AJ, Rodríguez-Perálvarez ML, Pageaux G, Sanchez-Fueyo A, Feng S. Liver transplantation immunology: Immunosuppression, rejection, and immunomodulation. Journal of Hepatology. 2023; 78(6):1199-215. [DOI:10.1016/j.jhep.2023.01.030] [PMID]
- Addissouky TA. Innovations in combined cardiac-liver transplantation: Robotic-assisted surgery and advanced organ preservation techniques. The Cardiothoracic Surgeon. 2025; 33(1):1-11. [DOI:10.1186/s43057-025-00158-0]
- Mauro E, Jutras G, Garcia R, Soler Perromat A, Llarch N, Holguin Arce V, et al. Challenges in liver transplantation for hepatocellular carcinoma: A review of current controversies. Cancers. 2023; 16(17):3059. [DOI:10.3390/cancers16173059] [PMID] [PMCID]
- Addissouky TA, El Tantawy El Sayed I, Wang Y. Epigenetic factors in posttraumatic stress disorder resilience and susceptibility. Egyptian Journal of Medical Human Genetics. 2025; 26:50. [DOI:10.1186/s43042-025-00684-w]
- Li W, Liu Z, Tang F, Jiang H, Zhou Z, Hao X, et al. Application of 3D Bioprinting in Liver Diseases. Micromachines. 2023; 14(8):1648. [DOI:10.3390/mi14081648] [PMID] [PMCID]
- Addissouky TA. Artemisinin and its derivatives throughout the therapeutic mechanisms and clinical potential. Discover Chemistry. 2025; 2:10. [DOI:10.1007/s44371-025-00084-4]
- Kumar S, Malviya R. Bioprinting of hepatic tissue using 3D technology: Transitioning beyond laboratory models to real-world applications in medical treatments. Applied Materials Today. 2024; 39:102307. [DOI:10.1016/j.apmt.2024.102307]
- Addissouky TA. From reactive to proactive: Advances leading the paradigm shift in cholecystitis management. Research in Molecular Medicine. 2023; 11(3):161-76. [DOI:10.32598/rmm.11.3.1265]
- Feng L, Wang Y, Fu Y, Li T, He G. Stem cell-based strategies: The future direction of bioartificial liver development. Stem Cell Reviews and Reports. 2024; 20(3):601-16. [DOI:10.1007/s12015-023-10672-5] [PMID]
- Zhang Y, Li L, Dong L, Cheng Y, Huang X, Xue B, et al. Hydrogel-based strategies for liver tissue engineering. Chem & Bio Engineering. 2024; 1(11):887-915. [DOI:10.1021/cbe.4c00079]
- Addissouky TA. Personalized pain pathways in liver fibrosis: Towards precision hepatology. Avicenna Journal of Medical Biochemistry. 2024; 12(2):162-71. [DOI:10.34172/ajmb.2535]
- Ietto G, Iori V, Gritti M, Inversini D, Costantino A, Izunza Barba S, et al. Multicellular liver organoids: Generation and importance of diverse specialized cellular components. Cells. 2023; 12(10):1429. [DOI:10.3390/cells12101429] [PMID] [PMCID]
- Addissouky TA. Next generation of colorectal cancer management: Integrating omics, targeted therapies, and smart technologies. Avicenna Journal of Medical Biochemistry. 2024; 12(2):131-43. [DOI:10.34172/ajmb.2530]
- Toprakhisar B, Verfaillie CM, Kumar M. Advances in recellularization of decellularized liver grafts with different liver (stem) cells: Towards clinical applications. Cells. 2023; 12(2):301. [DOI:10.3390/cells12020301] [PMID] [PMCID]
- Addissouky TA. Polyploidy-mediated resilience in hepatic aging: Molecular mechanisms and functional implications. Egyptian Liver Journal. 2024; 14(1):1-14. [DOI:10.1186/s43066-024-00391-y]
- Jakubowska M, Wisniewska MJ, Wencel A, Wojciechowski C, Gora M, Dudek K, et al. Hollow fiber bioreactor with genetically modified hepatic cells as a model of biologically active function block of the bioartificial liver. Biocybernetics and Biomedical Engineering. 2024; 44(1):9-19. [DOI:10.1016/j.bbe.2023.11.003]
- Somasekhar L, Griffiths LG. Current challenges and future promise for use of extracellular matrix scaffold to achieve the whole organ tissue engineering moonshot. Stem Cells Translational Medicine. 2023; 12(9):588-602. [DOI:10.1093/stcltm/szad046] [PMID] [PMCID]
- Liang J, Liu P, Yang X, Liu L, Zhang Y, Wang Q, et al. Biomaterial-based scaffolds in promotion of cartilage regeneration: Recent advances and emerging applications. Journal of Orthopaedic Translation. 2023; 41:54-62. [DOI:10.1016/j.jot.2023.08.006] [PMID] [PMCID]
- Qureshi AA, Wehrle CJ, Ferreira-Gonzalez S, Jiao C, Hong H, Dadgar N, et al. Tumor organoids for primary liver cancers: A systematic review of current applications in diagnostics, disease modeling, and drug screening. JHEP Reports. 2024; 6(12):101164. [DOI:10.1016/j.jhepr.2024.101164] [PMID] [PMCID]
- Afonso MB, Marques V, van Mil SWC, Rodrigues CMP. Human liver organoids: From generation to applications. Hepatology. 2024; 79(6):1432-51. [DOI:10.1097/HEP.0000000000000343] [PMID] [PMCID]
- Novelli G, Spitalieri P, Murdocca M, Centanini E, Sangiuolo F. Organoid factory: The recent role of the human induced pluripotent stem cells (hiPSCs) in precision medicine. Frontiers in Cell and Developmental Biology. 2023; 10:1059579. [DOI:10.3389/fcell.2022.1059579] [PMID] [PMCID]
- Wang J, Li Q, Li W, Méndez-Sánchez N, Liu X, Qi X. Stem cell therapy for liver diseases: Current perspectives. Frontiers in Bioscience-Landmark. 2023; 28(12):359. [DOI:10.31083/j.fbl2812359] [PMID]
- Liu S, Cheng C, Zhu L, Zhao T, Wang Z, Yi X, et al. Liver organoids: Updates on generation strategies and biomedical applications. Stem Cell Research & Therapy. 2024; 15(1):244. [DOI:10.1186/s13287-024-03865-3] [PMID] [PMCID]
- De Siervi S, Turato C. Liver organoids as an in vitro model to study primary liver cancer. International Journal of Molecular Sciences. 2023; 24(5):4529. [DOI:10.3390/ijms24054529] [PMID] [PMCID]
- Hendriks D, Brouwers JF, Hamer K, Geurts MH, Luciana L, Massalini S, et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nature Biotechnologyl. 2023; 41(11):1567-81. [DOI:10.1038/s41587-023-01680-4] [PMID] [PMCID]
- Geurts MH, Clevers H. CRISPR Engineering in organoids for gene repair and disease modelling. Nature Reviews Bioengineering. 2022; 1(1):32-45. [DOI:10.1038/s44222-022-00013-5]
- Khairnar R, Islam MA, Fleishman J, Kumar S. Shedding light on non-alcoholic fatty liver disease: Pathogenesis, molecular mechanisms, models, and emerging therapeutics. Life Sciences. 2023; 312:121185. [DOI:10.1016/j.lfs.2022.121185] [PMID]
- Doghish AS, Elballal MS, Elazazy O, Elesawy AE, Elrebehy MA, Shahin RK, et al. The role of miRNAs in liver diseases: Potential therapeutic and clinical applications. Pathology, Research and Practice. 2023; 243:154375. [DOI:10.1016/j.prp.2023.154375] [PMID]
- Addissouky TA, Sayed IE, Agroudy AE, Wang Y. Evaluating non-invasive biomarkers and composite scores for liver fibrosis diagnosis in hepatitis B and C infections. SN Comprehensive Clinical Medicine. 2025; 7(1):239. [DOI:10.1007/s42399-025-02005-z]
- Agarwal T, Banerjee D, Konwarh R, Esworthy T, Kumari J, Onesto V, et al. Recent advances in bioprinting technologies for engineering hepatic tissue. Materials Science & Engineering. C, Materials for Biological Applications. 2021; 123:112013. [DOI:10.1016/j.msec.2021.112013] [PMID]
- Addissouky TA, Sayed IE, Agroudy AE, Wang Y. Evaluating non-invasive biomarkers and composite scores for liver fibrosis diagnosis in hepatitis B and C infections. SN Comprehensive Clinical Medicine. 2025; 7(1):239. [DOI:10.1007/s42399-025-02005-z]
- De Siervi S, Turato C. Liver organoids as an in vitro model to study primary liver cancer. International Journal of Molecular Sciences. 2023; 24(5):4529. [DOI:10.3390/ijms24054529] [PMID] [PMCID]
- Hendriks D, Brouwers JF, Hamer K, et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nature Biotechnology. 2023; 41(11):1567-81. [DOI:10.1038/s41587-023-01680-4] [PMID] [PMCID]
- Aykora D, Taşçı B, Şahin MZ, Tekeoğlu I, Uzun M, Sarafian V, et al. Tendon regeneration deserves better: Focused review on In vivo models, artificial intelligence and 3D bioprinting approaches. Frontiers in Bioengineering and Biotechnology. 2025; 13:1580490. [DOI:10.3389/fbioe.2025.1580490] [PMID] [PMCID]
- Zhang Z, Zhou X, Fang Y, Xiong Z, Zhang T. AI-driven 3D bioprinting for regenerative medicine: From bench to bedside. Bioactive Materials. 2024; 45:201-30. [DOI:10.1016/j.bioactmat.2024.11.021] [PMID] [PMCID]
- Yi H, Lee G, Park S, Ha J, Choi D, Ko J, et al. Reconstructing the female reproductive system using 3D bioprinting in tissue engineering. Materials Today. Bio. 2025; 34:102127. [DOI:10.1016/j.mtbio.2025.102127] [PMID] [PMCID]
- Luce E, Messina A, Duclos-Vallée JC. Hepatic organoids as a platform for liver disease modeling and the development of novel therapies. Clinics and Research in Hepatology and Gastroenterology. 2025; 49(7):102647. [DOI:10.1016/j.clinre.2025.102647] [PMID]
- Luce E, Duclos-Vallee JC. Stem cells and organoids: A paradigm shift in preclinical models toward personalized medicine. Pharmaceuticals). 2025 1; 18(7):992. [DOI:10.3390/ph18070992] [PMID] [PMCID]
- Rezvani M. Human liver immunology: From in vitro models to new insights. Cellular & Molecular Immunology. 2025. [DOI:10.1038/s41423-025-01312-8] [PMID]
- Franco C. From flat to spatial: Transitioning from 2D to 3D in vitro models for nicotine based oxidative stress and lung granuloma formation [PhD dissertation]. Brescia: Università degli Studi di Brescia; 2025.
Type of Study: Review Article |
Subject:
Health
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









