Volume 10, Issue 4 (Autumn 2022)
Iran J Health Sci 2022, 10(4): 35-50 |
Back to browse issues page
Ethics code: 3818-95-4
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ramzani Ghara A, Ezzati Ghadi F, Mousaie A. Acute Oral Toxicity Study of Indigofera Tinctoria L. Aqueous Extract, a Substitute for Synthetic Food Colorants: Fourier Transform Infrared Spectroscopy, Biochemical, and Histopathological Investigation. Iran J Health Sci 2022; 10 (4) :35-50
URL: http://jhs.mazums.ac.ir/article-1-825-en.html
URL: http://jhs.mazums.ac.ir/article-1-825-en.html
Department of Biology, Faculty of Science, University of Jiroft, Jiroft, Iran. , a.ramzani@ujiroft.ac.ir
Keywords: Acute oral toxicity, Fourier transform infrared spectroscopy, Histopathology, Indigofera tinctoria extract
Full-Text [PDF 3179 kb]
(1354 Downloads)
| Abstract (HTML) (2732 Views)
Full-Text: (1574 Views)
1. Introduction
Over the past few years, the global market of the food colors industry has experienced rapid growth. Much attention is paid to the toxicity of synthetic additives used in the food industry. The use of natural food pigments has risen owing to the growing awareness of environmental risks and the side-effect of chemicals in synthesizing food colorants [1].
According to the World Health Organization, 80% of the world’s population uses natural products for primary health care [2]. Iran has a small share of these plants in the global trade market. The area under cultivation for medicinal plants in Iran was about 605876 hectares in 2017. Of these lands, 262428 tons of mainly henna, cumin, coriander, fennel, damask rose, and indigo was harvested [3]. The Iranian national standard organization only permits seven artificial dyes of Quinoline Yellow (E104), Sunset Yellow FCF (E110), Azorubine (E122), Ponceau 4R (E124), Allura Red AC (E129), Indigotine (E132), and Brilliant Blue FCF (E133) in a different type of products [4].
Nevertheless, some synthetic color additives may present health problems, namely allergenic problems, hyperactivity in children, and carcinogenic pathologies [5, 6]. Alternatively, consuming natural compounds has therapeutic effects on several diseases, such as cancer, chronic bronchitis, epilepsy, neuropathy, asthma, ulcers, and diuretic [7]. Therefore, identifying the bioactive compounds of medicinal plants and studying their effects on humans and animals is of great importance [8].
The genus Indigofera contains certain economically significant indigo dye-producing species, such as I. tinctoria and I. suffruticosa [9]. I. tinctoria belongs to the family of the Fabaceae, which is distributed across various tropical regions. This plant is cultivated in Southern Iran, especially in Jiroft County, Kerman Province. It is a deciduous shrub that reaches a height of 1-2 m, which may be annual, biennial, or perennial. It has been used as a source of dyeing agent, i.e., indigo, since ancient times. In 1986, Indigo was cultivated on about 1694 hectares in Iran, which decreased to 520 hectares in 2006 [10]. At present, Kerman Province, with about 300 hectares of indigo cultivation area, has one of the most important summer crops.
The herb is traditionally used for neurological disorders, epilepsy, bronchitis, and hepatic disease [11]. Srinivasan et al. (2016) reported that I. tinctoria leaves contained phenols, flavonoids, saponins, and terpenoids in their aqueous extract [12]. I. tinctoria generates a high-quality dye compared to other plants and is also referred to as true indigo [13]. The indigo precursor in I. tinctoria is indicant, found mainly in the leaves with content ranging 0.2%-0.7% [14]. Water extract of I. tinctoria is known to contain three major components: indican, indigo, and its isomer of indirubin [15]. It is well known that indican hydrolysis to indoxyl and further oxidation of indoxyl can be formed into indigo and indirubin [16]. Indirubin has anti-inflammatory [17], anti-cell proliferative [18], antioxidant [19], anti-diabetic, anti-dyslipidaemic [20], and wound-healing properties [21]. It has been shown that indigotin, a colorless glycoside producing the blue color dye, possesses antiseptic and astringent properties [22]. However, no systemic toxicological data on I. tinctoria leaf powder has been reported in the literature [9]. In contrast, the neuroprotective role of aqueous extract of I. tinctoria was reported in Wistar rats [23].
Similarly, a study on Wistar rats using aqueous extracts of I. tinctoria revealed its immunoprotective functions against chronic noise stress [24]. However, conducting further research using precise and modern analytical techniques can help researchers find better the likely beneficial or detrimental effects of this plant on human and animals. Spectroscopy has recently emerged as a key tool for biomedical applications and made significant advances in clinical diagnosis due to its low cost, as well as rapid, simple, and convenient use [25]. Fourier transform infrared spectroscopy (FTIR) is a physicochemical and non-destructive analytical technique that can provide detailed chemical information on the sample composition [26]. F-IR is a type of vibrational spectroscopy that identified structural moieties of bio-molecules based on their IR absorption. This technique can be used to analyze bio-molecules, including carbohydrates, proteins, nucleic acids, and lipids, with a minimum quantity of samples [27]. Because of the limited data regarding the oral toxicity of I. tinctoria in humans and animals, this study was carried out to evaluate the likely oral toxicity of I. tinctoria extract by FTIR spectroscopy combined with histopathological and serum biochemical assays in Wistar rats.
2. Materials and Methods
The liver enzymes of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and triglyceride (TG) and high-density lipoprotein (HDL) test kits were purchased from Pars Azmoon Co., Iran. All applied chemicals and reagents were of analytical grade. Deionized and distilled water was used throughout.
The plant samples were collected from Jiroft County, Iran. Geographically, it is located at coordinates 28˚°40ˊ 41.0052˝ N-57˚ 44ˊ 25.9980˝ E. The plant was authenticated by the Department of Biology, University of Jiroft. Voucher specimens were deposited at the Herbarium of the plant biology laboratory (Department of Biology, University of Jiroft; Reg No.: 312). The collected plants were dried in the shade at room temperature (25°C±2°C). The leaves were ground to coarse powder with an appropriate grinder. The powder was stored in a dark, dry, and cool place.
A total of 5g of the ground leaves was extracted with distilled water (100mL) in a conical flask by the maceration method. Briefly, the mixture was shaken with the laboratory shaker at a speed of 120 rpm for 2h and kept at 4°C for 72h. The obtained aqueous extract was filtered using a Whatman filter paper No.1. The filtrates were then concentrated through a rotary evaporator under reduced pressure and then stored at 4°C for further use [16].
Twenty-five male Wistar rats with Mean±SD initial body weight of 180±20g were purchased from the Razi Institute of Kerman, Iran. The rats were housed and handled in a standard condition with a 12h light/dark cycle and temperature of approximately 22°C for at least one week before the experiments. The animals were housed in a propylene cage with free access to standard pellets (Javaneh Khorasan Co., Mashhad, Iran) ad libitum. All research procedures were approved by the Internal Committee of Animals’ ethics and care of the University of Jiroft. The sub-chronic toxicity study was done according to the OECD Guidelines No. 407. The experimental animals were randomly selected and divided into five groups as follows:
Group I: Normal control rats;
Group II: Oral administration of I. tinctoria extract (100mg/kg Body Weight [BW]);
Group III: Oral administration of I. tinctoria extract (250mg/kg BW);
Group IV: Oral administration of I. tinctoria extract (500mg/kg BW);
Group V: Oral administration of I. tinctoria extract (1000mg/kg BW);
The I. tinctoria extract at different concentrations was administrated orally to the rats daily for 14 days. The rat’s body weight was recorded weekly, and the quantity of administered I. tinctoria extract was adjusted weekly based on the new body weight to ensure a constant dose volume per kg body weight during the experimental period.
Animals were observed during the first two hours after aqueous extract administration and supplied with food. The animal’s mortality was recorded after 24h. The observations included a response to treatment, skin and eye changes, sleep, diarrhea, and coma. Each cage was supplied with the required standard pellet as food and water. Each group was received 100g standard pellet ad libitum every day. The daily food (g/d) and water (mL/d) consumption were recorded on days 7 and 14 of the experiment. At the end of the experimental period, the overnight fasted rats were anesthetized with diethyl ether, and blood samples were taken by cardiac puncture. ALT, AST, HDL, TG, and serum glucose concentrations were quantified by an autoanalyzer (Hitachi 912, Japan).
To detect the possible toxicity of extract doses to rats, the histological examination of various tissues was done by fixation of tissues in 10% formalin, and the tissue sections (5μm) were stained with Hematoxylin and Eosin [28].
The tissue samples of each group were dissected and dried in an oven at 70°C. The dried samples were made to fine powder by grinder apparatus and then were pelleted using KBr. The spectra were measured in the range of 4000-400cm-1.
The statistical analysis was done by a Statistical Analysis System (SAS, v. 9.2) followed by the least significant difference (LSD) mean comparison test of the software. The results were considered statistically significant if P<0.05. Excel software was used to compare the data of FTIR spectroscopy from different treated groups. All given values were expressed as the mean and standard error of means (SEM).
3. Results
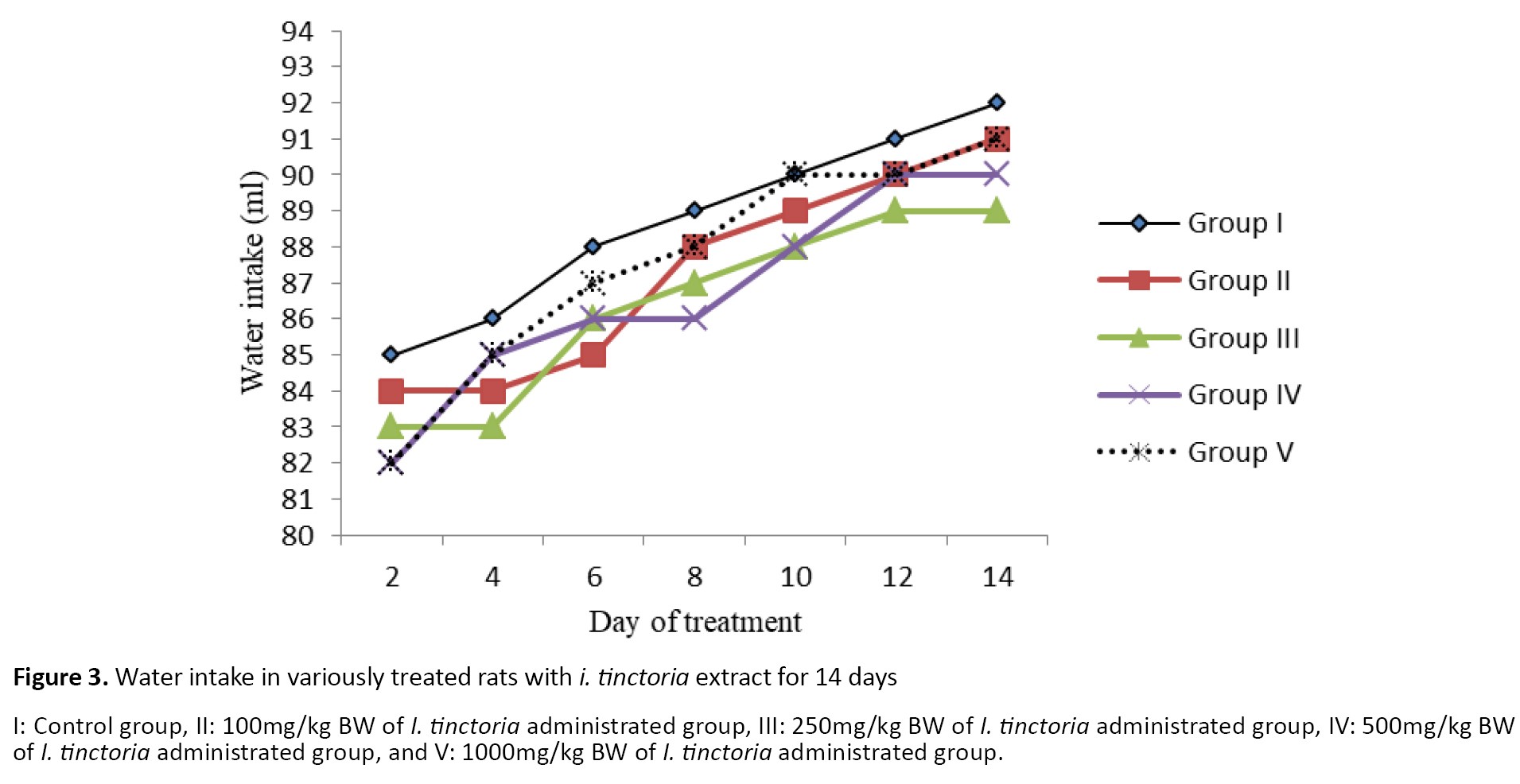
Results indicated no mortality and behavioral differences among experimental groups throughout the study. Accordingly, based on the findings, up to a dose of 1g/kg BW was safe and healthy in Wistar rats. The Mean±SD initial BW of control rats was 191.60±7.92g. As shown in Figure 1, rats’ body weights gradually increased during the experimental period. According to the result, no significant differences were detected in body weights between the control and treatment groups at any time during the research. The food consumption and water intake of all treated rats are presented in Figures 2 and 3. The experimental treatments had no influence on the food and water consumption of rats during the experiment. The results of the blood metabolites following I. tinctoria administration (14 days) are shown in Table 1.
.jpg)
In the present study, 100mg/kg I. tinctoria-treated rats showed lower ALT activities than the control rats (P<0.05). There was no difference between the other groups.
The current investigation showed no difference in serum urea concentration between treatments. Only group V (1000 mg/kg) with III (250 mg/kg) differences was close to significant (P=0.06). In the current study, 100, 250, 500, and 1000mg/kg of I. tinctoria administration had no effects on the serum HDL of rats compared to the control group. In addition, the results revealed that triglyceride concentrations in group II (100 mg/kg) were lower than in the control and groups III and IV groups (P<0.05).
According to the results, no difference in blood glucose concentration was observed between the groups receiving the extract and the control group (P>0.05). However, blood glucose concentrations in rats that received 100mg/kg BW of the extract were lower than that in group IV (P<0.05). The administration of the aqueous extract at the doses of 100, 250, 500, and 1000mg/kg led to no changes in total protein.
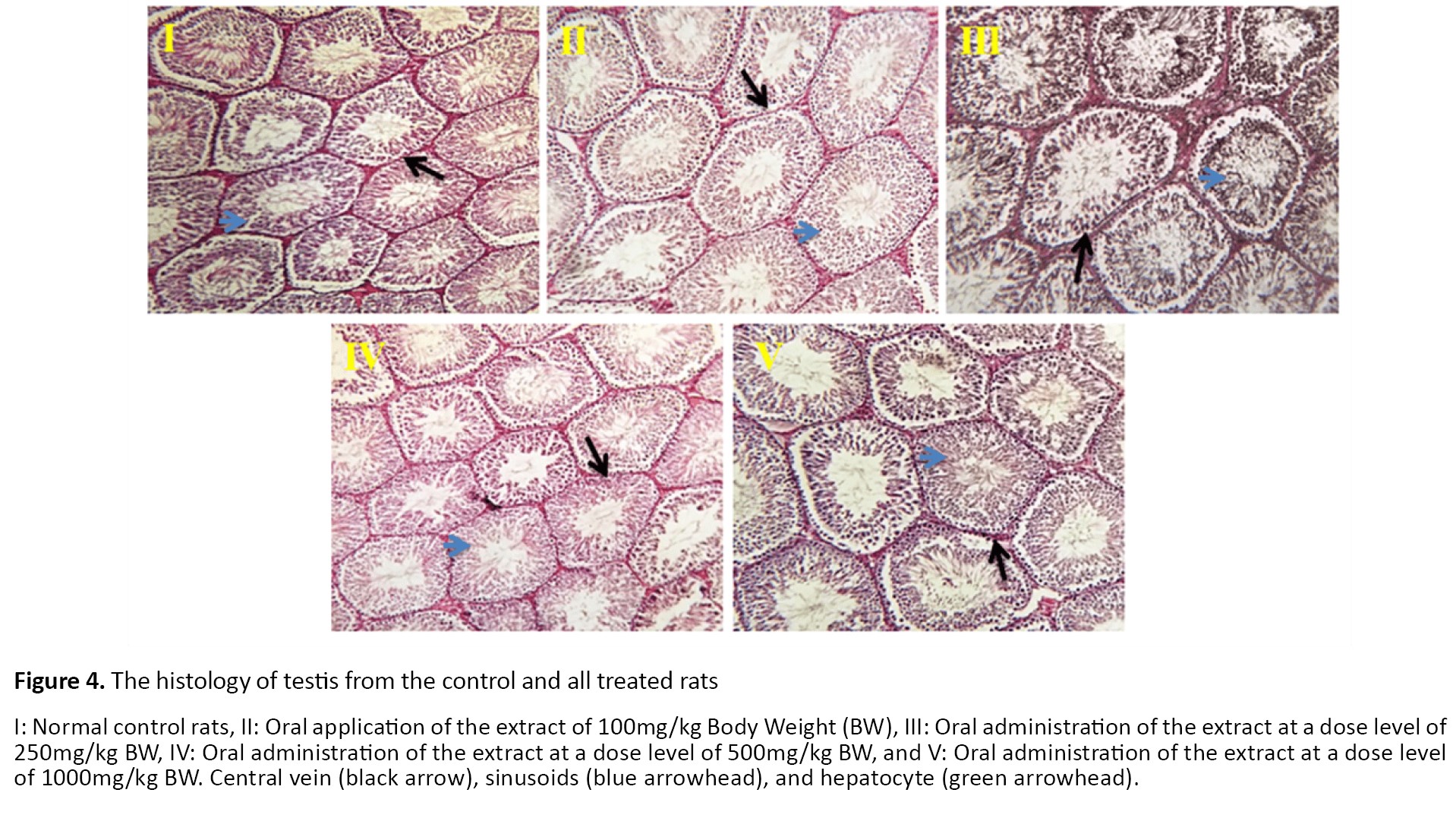
The histopathological analysis of rats’ testes tissue is shown in Figure 4. Animals’ testes showed normal architecture and well-organized germ cells. All treated groups revealed normal seminiferous tubules with regular arrangements of cells.
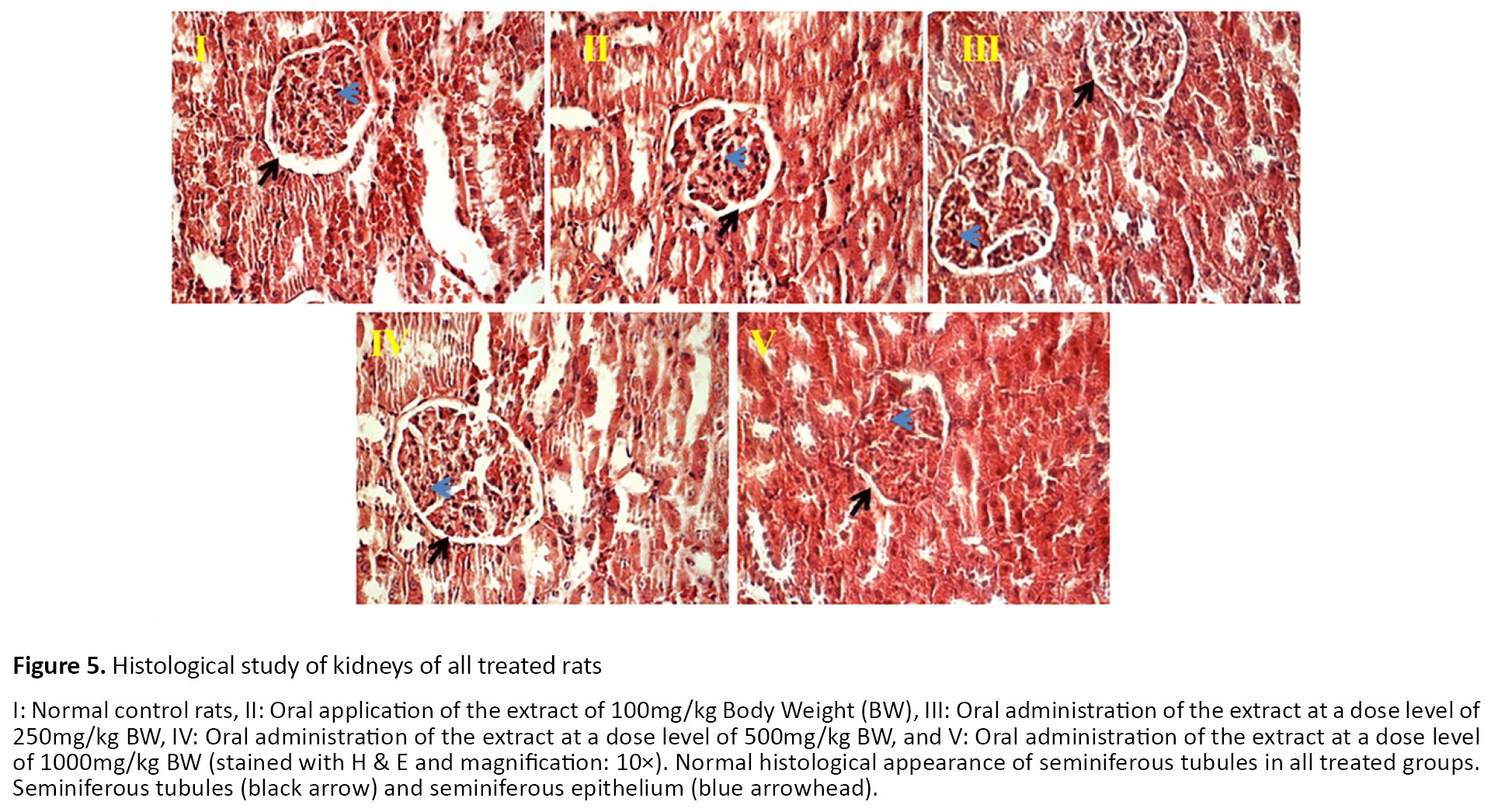
Photomicrographs from the kidney tissues of rats are presented in Figure 5. The kidney of the control group revealed a normal structure of glomeruli and proximal and distal tubules. Also, normal distal and proximal tubules were seen in all treated groups.
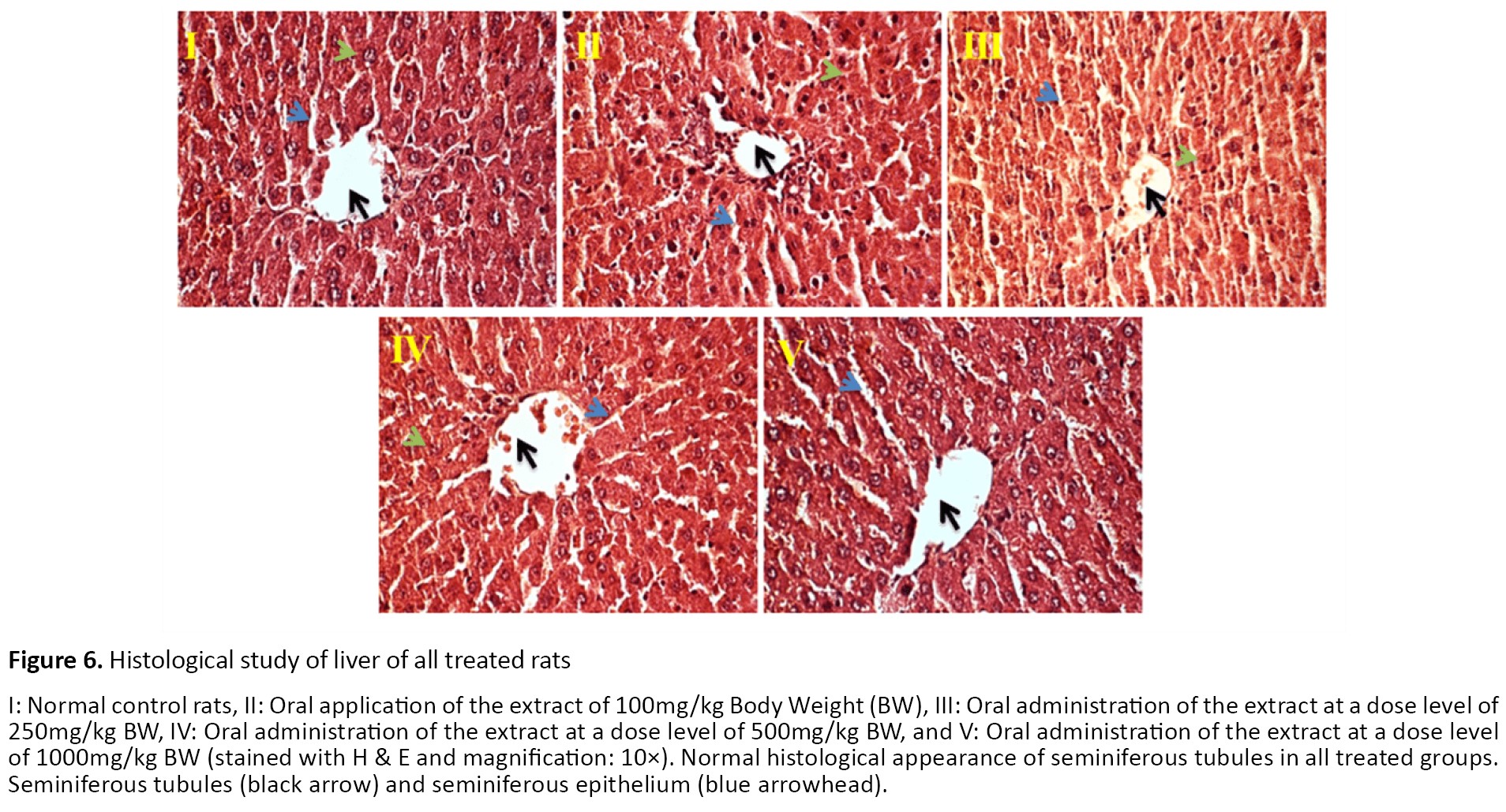
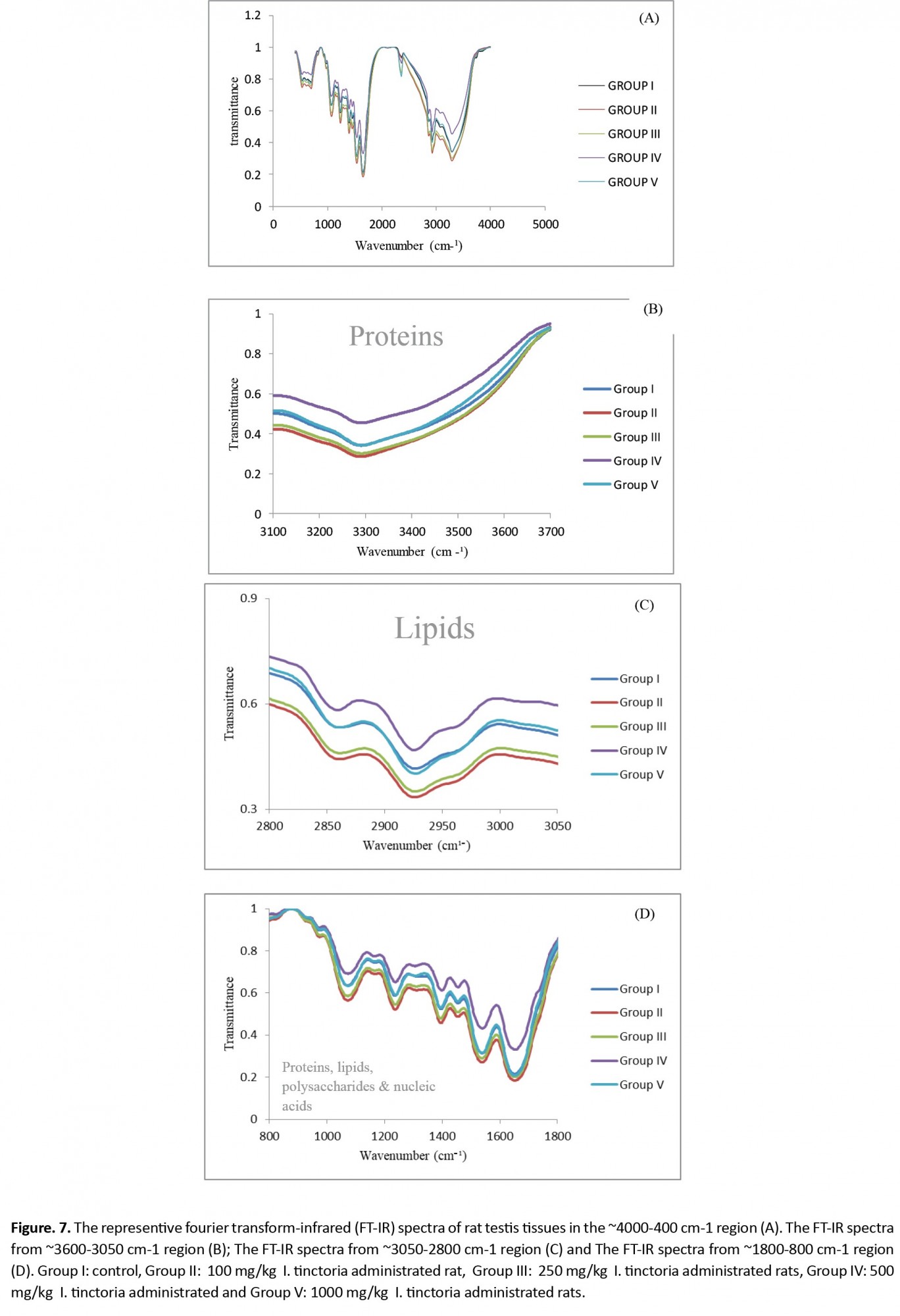
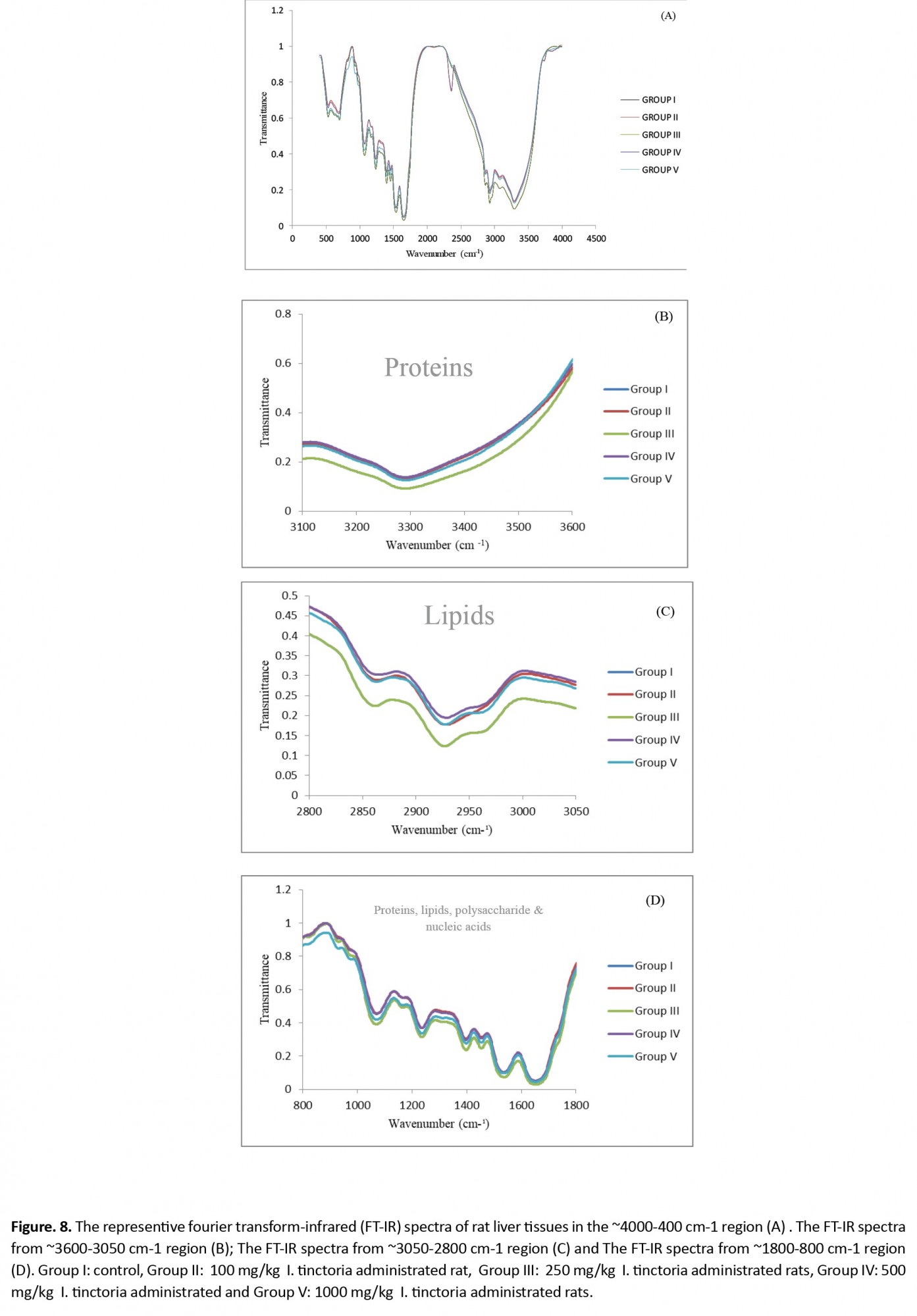
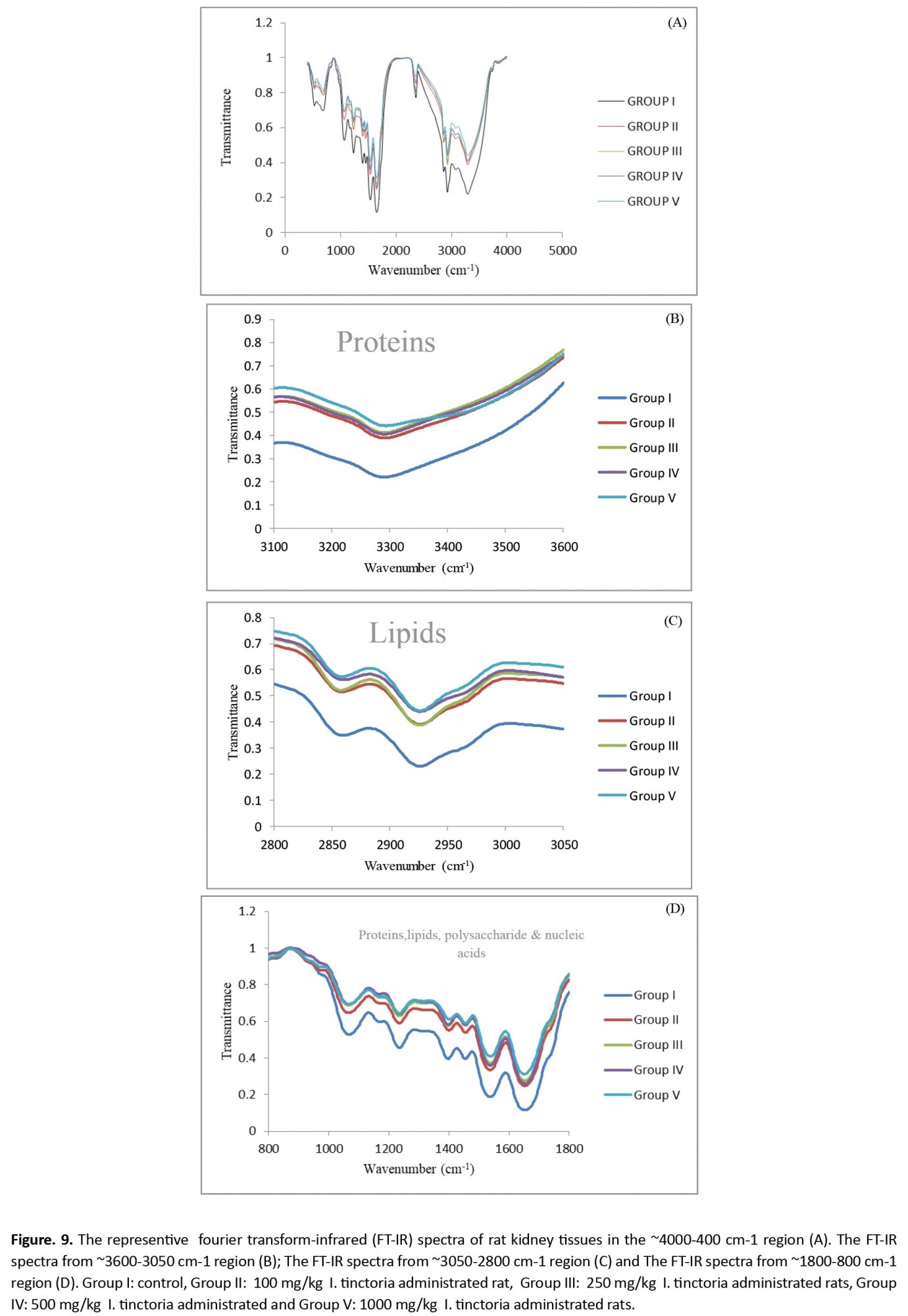
Histopathological observation of liver tissues from the control and extract-treated groups showed normal architecture (Figure 6). The FTIR absorption spectra between ~4000 cm-1 and ~400 cm-1 and the peak assignments of the testis, liver, and kidney tissues are shown in Figures 7-9 and presented in Tables 3-5.
.jpg)
The results of testes FTIR (Figure 7 and Table 2) indicated that three bands at ~2360 cm-1, ~696 cm-1, and ~530 cm-1 were missing in rats that received 100 mg/kg of the extract. Bands at 696 and 530 cm-1 are mainly due to nucleic acid.
.jpg)
Also, the detailed spectral analysis was done in three distinct frequency ranges, namely ~3600-3050 cm-1 (Figure 7B, 8B, and 9B), ~3050-2800 cm-1 (Figure 7C, 8C, and 9C), and ~1800-800 cm-1 (Figure 7D, 8D, and 9D) to determine the details of macromolecules changes. The peaks between ~3600 and 3050 cm-1 regions mainly consist of amide-A vibrations of proteins. The band appearing at ~3292 cm-1 in the control group did not significantly change in other treated groups (Table 2 and Figure 7B).
Figure 7C shows that the region from ~3050 to ~2800 cm-1 belongs to lipids of control and various treated groups. The bands are primarily due to CH2 asymmetric and symmetric stretching in the testis tissue. The results (Table 2 and Figure 7B) revealed that two peaks in the region of ~2926 and ~2868 cm-1 had not changed in all treated groups compared to the control.
Figure 7D shows that absorptions are due to the continuation of polysaccharides, nucleic acids, phospholipids, and carbohydrates in the range of ~1800 to ~800 cm-1. The main band at ~1235 cm-1 was because of the asymmetric stretching vibration of phospholipids, and the band observed at ~1071 cm-1 was related to PO2−asymmetric stretching: mainly nucleic acids.
According to the results presented in Tables 3 and 4, all absorption frequencies were the same in various treated groups.
.jpg)
However, the two bands at 2357 and 681 cm-1 were missing in group II kidney and liver tissues (Figure 8A, 8D, and 9A, 9B) that belong to lipids and nucleic acids. Results from Table 5 show band area ratios of amide-II/amide-I and amide-II/amide-A that indicate the protein rearrangement.
Tables 3, 4, and 5 and Figure 7A, Figure 8A, and Figure 9A show the area value of amide-I, amide-II, and amide-A, which are responsible for the secondary structure of proteins in the testis, liver, and kidney, respectively. 4. Discussion
The tendency to use natural dyes has increased worldwide [29]. The blue color of I. tinctoria is one of the oldest dyes known to humans. Indigo has an attractive blue color, high color stability, and high strength to combine with different natural colors. In the world, indigo is widely used as a medicinal plant. In Iran, the leaves of this plant are used to treat foot pain, burns, and food cracks. Also, antioxidant, anti-diabetic, anti-inflammatory, and anti-dyslipidemic properties of this plant have been reported. In the present study, oral toxicity of I. tinctoria extract was evaluated by FTIR spectroscopy combined with histopathological and serum biochemical assays in Wistar rats. Rats were divided into five groups (n=5 for each group). Group I served as the control, and the other four groups received respectively 100mg/kg (Group II), 250mg/kg (Group III), 500mg/kg (Group IV), and 1000mg of I. tinctoria per kg BW (Group V) for 14 days. The normality of data was determined using the Shapiro-Wilk normality test, all data were normal with homogen variances, and then, data were analyzed using the GLM procedure of the software.
According to the findings in the present study, the experimental treatments had no influence on the food and water consumption of rats during the experiment. Aylward et al. reported that Indigofera species did not change growth rates relative to control feeds [30]. Kumar et al. reported that animals treated with an aqueous extract of I. aspalathoides, a species of Indigofera, at a dose of 250mg/kg BW, did not alter the food and water intake [17]. Moreover, they suggested that the plant extract had no toxic side effects. A study also reported that the chemopreventive effect of I. aspalathoides and no sub-acute toxicity was observed [31]. Therefore, no alteration in BW, as well as feed and water intake of rats, may imply the nontoxic effects of I. tinctoria on these animals.
To the best of our knowledge, the toxic effect of I. tinctoria on testes, liver, and kidney, and biochemical and biophysical parameters of animal models, has not been investigated. The liver is an important body organ in metabolism and detoxification. Liver enzyme activities such as ALT and AST indicate liver injury, as enzymes leaking from liver cells cytosol into the bloodstream correlate with diseases such as diabetes, toxicant, and inflammatory processes of the liver [32, 33]. In the present study, 100mg/kg I. tinctoria-treated rats showed significant decrease of ALT level (P<0.05) with compared to control rats. There was no difference between the other groups. This finding indicates the plant hepatoprotective effect at a dose level of 100mg/kg [34]. Inconsistent with our results, Muthulingam et al. studied the impact of I. tinctoria on paracetamol-induced liver damage in rats and showed decreased ALT levels in 250 and 500mg/kg I. tinctoria-treated rats [35].
Serum creatinine and urea are the major indicators of kidney failure. The increased levels of creatinine and urea have been reported in gentamicin-induced nephrotoxicity in rats [36]. The current study showed no difference in serum urea concentration between treatments. Moreover, the serum urea concentration of 1000mg/kg and 250mg/kg of plant administration was close to significant (P=0.06). The liver also contributes to the synthesis of lipoproteins and the metabolism of cholesterol. An increase in plasma lipids, particularly cholesterol, is a common feature of diseases, such as heart disease and diabetes [37]. A study confirmed that the changes in plasma lipids could serve as a simple indicator for assessing liver disorders [38]. In the current study, I. tinctoria administration had no effects on the serum HDL of rats compared to the control group. In addition, the results revealed that triglyceride concentrations in group II (100mg/kg) were lower than in the control and groups III and IV groups (P<0.05). Tsuji et al. reported that rats’ treatment with the indigo plant (Polygonum tinctorium Lour) improved lipid profiles and suggested its application as a food supplement [39]. We found that I. tinctoria regulates lipid profiles, including TG and HDL. To our knowledge, this is the first study showing that I. tinctoria regulates lipid metabolism in vivo.
In the present study, blood glucose levels were estimated. Results indicated that 100mg/kg BW of the extract was more effective than 500mg/kg in lowering glucose concentration. Studies on the leaves of the genus Indigofera, i.e., I. tinctoria, I. affect, and I. pluchra, indicate the presence of natural products that produce hypoglycemic effects [40, 41].
Protein plays a critical role in interactions between the intra- and extra-cellular media of animal cells. Proteins are of great importance in the synthesis of microsomal detoxifying enzymes and detoxifying the toxicants that enter the body [42]. The results show that, the administration of different doses of the aqueous extract led to no changes in total protein.
The reproductive system is one of the most susceptible body organs to toxic agents. Then, the study of its response to each xenobiotic or external treatment is very important. To determine the toxic oral effect of I. tinctoria on the reproductive functions of healthy male rats, we studied the histopathological status of the testis. In the present study, no abnormalities were observed in I. tinctoria-treated rat testis. It can be explained due to the antioxidant activity of I. tinctoria.
Sreepriya et al. reported the antioxidant activity of I. tinctoria, which is very effective in oxidative stress-induced liver damage [43]. The control rats’ livers showed that the hepatic cells are radially placed, and each cell has a large spherical nucleus and granular cytoplasmic structure without any injury. Dkhil et al. reported that Indigofera oblongifolia fights against hepatic injury. The hepatoprotective effect of Indigofera can be explained due to its antioxidant activities [44]. It was shown that Indigofera oblongifolia was successful in preventing lead acetate accumulation in the liver and kidneys [45]. In the present study, biochemical results were supported by histopathological data.
The areas of the absorption bands in the FTIR spectrum are directly associated with the concentration of the molecules [46]. The results of testes FTIR showed that three bands region at ~2360 cm-1, ~696 cm-1, and ~530 cm-1 were missing in rats that received 100mg/kg of the extract. Stuart reported that a peak around ~2300 cm-1 was due to the CH-stretching of lipids [47]. Raouf et al. studied the neurons of the rat brain by using FTIR spectroscopy. They reported that the peaks between ~1000 to ~400 cm-1 regions belong to nucleic acids [48].
In the current study, according to the findings, the testes lipids and nucleic acids of group II have changed; however, no changes were observed in other I. tinctoria-treated animals. Also, the detailed spectral analysis was done in three distinct frequency ranges, to determine the details of macromolecules changes. The peaks between ~3600 and 3050 cm-1 regions mainly consist of amide-A vibrations of proteins. The band appearing at ~3292 cm-1 in the control group did not significantly change in other treated groups. This finding indicates that the protein content did not change due to various doses administered to the rats.
The main band at ~1235 cm-1 and ~1071 cm-1 were because of the asymmetric stretching vibration of phospholipids, and related to PO2− asymmetric stretching: mainly nucleic acids, respectively. Results obtained from serum lipids profiles showed that triglyceride level was decreased in 100 mg/kg of I. tinctoria administrated rats in accordance with the lipid changes in FTIR result. However, there is limited information in the literature, and further studies using FTIR are required to investigate the likely impacts of I. tinctoria extract on different body organs of model animals.
The band ratios area of amide-II/amide-I and amide-II/amide-A had not changed in any treated groups. The results show that I. tinctoria does not affect the α-helix and β-sheet secondary structure of proteins in all treated rats compared to the control group.
5. Conclusion
In recent years, the tendency to use natural colors is rising due to the side effects of some synthetic colors. Along with the growing public awareness of the dangers posed by using synthetic colors, people are returning to using natural colors that are more environmentally friendly. In the present study, oral toxicity of I. tinctoria as a source of natural color was evaluated. According to results obtained from biochemical and histopathological assays, the aqueous extract of I. tinctoria is nontoxic and safe at doses of 100, 250, 500, and 1000mg/kg BW. Moreover, FTIR findings revealed that 100mg/kg BW administration of extract caused changes at the molecular level, which requires further studies. Further investigation into its medicinal and therapeutic efficacy could also be considered.
Study limitations
The study’s major limitation is that the exact mechanism of action of I. tinctoria extract could not be explained. Therefore, further research may be needed to support our results.
Ethical Considerations
Compliance with ethical guidelines
All research procedures were approved (3818-95-4) by the Internal Committee of Animals’ Ethics and Care of the University of Jiroft. The subchronic toxicity study was done according to the OECD Guidelines No. 407.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Supervision, Writing-review, editing, and conceptualization: Abdollah Ramzani Ghara; Writing original draft, and investigation: Fereshteh Ezzati Ghadi; Investigation, data analysis, writing, and editing: Amir Mousaie.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We are thankful to the University of Jiroft for supporting this work.
References
Over the past few years, the global market of the food colors industry has experienced rapid growth. Much attention is paid to the toxicity of synthetic additives used in the food industry. The use of natural food pigments has risen owing to the growing awareness of environmental risks and the side-effect of chemicals in synthesizing food colorants [1].
According to the World Health Organization, 80% of the world’s population uses natural products for primary health care [2]. Iran has a small share of these plants in the global trade market. The area under cultivation for medicinal plants in Iran was about 605876 hectares in 2017. Of these lands, 262428 tons of mainly henna, cumin, coriander, fennel, damask rose, and indigo was harvested [3]. The Iranian national standard organization only permits seven artificial dyes of Quinoline Yellow (E104), Sunset Yellow FCF (E110), Azorubine (E122), Ponceau 4R (E124), Allura Red AC (E129), Indigotine (E132), and Brilliant Blue FCF (E133) in a different type of products [4].
Nevertheless, some synthetic color additives may present health problems, namely allergenic problems, hyperactivity in children, and carcinogenic pathologies [5, 6]. Alternatively, consuming natural compounds has therapeutic effects on several diseases, such as cancer, chronic bronchitis, epilepsy, neuropathy, asthma, ulcers, and diuretic [7]. Therefore, identifying the bioactive compounds of medicinal plants and studying their effects on humans and animals is of great importance [8].
The genus Indigofera contains certain economically significant indigo dye-producing species, such as I. tinctoria and I. suffruticosa [9]. I. tinctoria belongs to the family of the Fabaceae, which is distributed across various tropical regions. This plant is cultivated in Southern Iran, especially in Jiroft County, Kerman Province. It is a deciduous shrub that reaches a height of 1-2 m, which may be annual, biennial, or perennial. It has been used as a source of dyeing agent, i.e., indigo, since ancient times. In 1986, Indigo was cultivated on about 1694 hectares in Iran, which decreased to 520 hectares in 2006 [10]. At present, Kerman Province, with about 300 hectares of indigo cultivation area, has one of the most important summer crops.
The herb is traditionally used for neurological disorders, epilepsy, bronchitis, and hepatic disease [11]. Srinivasan et al. (2016) reported that I. tinctoria leaves contained phenols, flavonoids, saponins, and terpenoids in their aqueous extract [12]. I. tinctoria generates a high-quality dye compared to other plants and is also referred to as true indigo [13]. The indigo precursor in I. tinctoria is indicant, found mainly in the leaves with content ranging 0.2%-0.7% [14]. Water extract of I. tinctoria is known to contain three major components: indican, indigo, and its isomer of indirubin [15]. It is well known that indican hydrolysis to indoxyl and further oxidation of indoxyl can be formed into indigo and indirubin [16]. Indirubin has anti-inflammatory [17], anti-cell proliferative [18], antioxidant [19], anti-diabetic, anti-dyslipidaemic [20], and wound-healing properties [21]. It has been shown that indigotin, a colorless glycoside producing the blue color dye, possesses antiseptic and astringent properties [22]. However, no systemic toxicological data on I. tinctoria leaf powder has been reported in the literature [9]. In contrast, the neuroprotective role of aqueous extract of I. tinctoria was reported in Wistar rats [23].
Similarly, a study on Wistar rats using aqueous extracts of I. tinctoria revealed its immunoprotective functions against chronic noise stress [24]. However, conducting further research using precise and modern analytical techniques can help researchers find better the likely beneficial or detrimental effects of this plant on human and animals. Spectroscopy has recently emerged as a key tool for biomedical applications and made significant advances in clinical diagnosis due to its low cost, as well as rapid, simple, and convenient use [25]. Fourier transform infrared spectroscopy (FTIR) is a physicochemical and non-destructive analytical technique that can provide detailed chemical information on the sample composition [26]. F-IR is a type of vibrational spectroscopy that identified structural moieties of bio-molecules based on their IR absorption. This technique can be used to analyze bio-molecules, including carbohydrates, proteins, nucleic acids, and lipids, with a minimum quantity of samples [27]. Because of the limited data regarding the oral toxicity of I. tinctoria in humans and animals, this study was carried out to evaluate the likely oral toxicity of I. tinctoria extract by FTIR spectroscopy combined with histopathological and serum biochemical assays in Wistar rats.
2. Materials and Methods
The liver enzymes of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and triglyceride (TG) and high-density lipoprotein (HDL) test kits were purchased from Pars Azmoon Co., Iran. All applied chemicals and reagents were of analytical grade. Deionized and distilled water was used throughout.
The plant samples were collected from Jiroft County, Iran. Geographically, it is located at coordinates 28˚°40ˊ 41.0052˝ N-57˚ 44ˊ 25.9980˝ E. The plant was authenticated by the Department of Biology, University of Jiroft. Voucher specimens were deposited at the Herbarium of the plant biology laboratory (Department of Biology, University of Jiroft; Reg No.: 312). The collected plants were dried in the shade at room temperature (25°C±2°C). The leaves were ground to coarse powder with an appropriate grinder. The powder was stored in a dark, dry, and cool place.
A total of 5g of the ground leaves was extracted with distilled water (100mL) in a conical flask by the maceration method. Briefly, the mixture was shaken with the laboratory shaker at a speed of 120 rpm for 2h and kept at 4°C for 72h. The obtained aqueous extract was filtered using a Whatman filter paper No.1. The filtrates were then concentrated through a rotary evaporator under reduced pressure and then stored at 4°C for further use [16].
Twenty-five male Wistar rats with Mean±SD initial body weight of 180±20g were purchased from the Razi Institute of Kerman, Iran. The rats were housed and handled in a standard condition with a 12h light/dark cycle and temperature of approximately 22°C for at least one week before the experiments. The animals were housed in a propylene cage with free access to standard pellets (Javaneh Khorasan Co., Mashhad, Iran) ad libitum. All research procedures were approved by the Internal Committee of Animals’ ethics and care of the University of Jiroft. The sub-chronic toxicity study was done according to the OECD Guidelines No. 407. The experimental animals were randomly selected and divided into five groups as follows:
Group I: Normal control rats;
Group II: Oral administration of I. tinctoria extract (100mg/kg Body Weight [BW]);
Group III: Oral administration of I. tinctoria extract (250mg/kg BW);
Group IV: Oral administration of I. tinctoria extract (500mg/kg BW);
Group V: Oral administration of I. tinctoria extract (1000mg/kg BW);
The I. tinctoria extract at different concentrations was administrated orally to the rats daily for 14 days. The rat’s body weight was recorded weekly, and the quantity of administered I. tinctoria extract was adjusted weekly based on the new body weight to ensure a constant dose volume per kg body weight during the experimental period.
Animals were observed during the first two hours after aqueous extract administration and supplied with food. The animal’s mortality was recorded after 24h. The observations included a response to treatment, skin and eye changes, sleep, diarrhea, and coma. Each cage was supplied with the required standard pellet as food and water. Each group was received 100g standard pellet ad libitum every day. The daily food (g/d) and water (mL/d) consumption were recorded on days 7 and 14 of the experiment. At the end of the experimental period, the overnight fasted rats were anesthetized with diethyl ether, and blood samples were taken by cardiac puncture. ALT, AST, HDL, TG, and serum glucose concentrations were quantified by an autoanalyzer (Hitachi 912, Japan).
To detect the possible toxicity of extract doses to rats, the histological examination of various tissues was done by fixation of tissues in 10% formalin, and the tissue sections (5μm) were stained with Hematoxylin and Eosin [28].
The tissue samples of each group were dissected and dried in an oven at 70°C. The dried samples were made to fine powder by grinder apparatus and then were pelleted using KBr. The spectra were measured in the range of 4000-400cm-1.
The statistical analysis was done by a Statistical Analysis System (SAS, v. 9.2) followed by the least significant difference (LSD) mean comparison test of the software. The results were considered statistically significant if P<0.05. Excel software was used to compare the data of FTIR spectroscopy from different treated groups. All given values were expressed as the mean and standard error of means (SEM).
3. Results
Results indicated no mortality and behavioral differences among experimental groups throughout the study. Accordingly, based on the findings, up to a dose of 1g/kg BW was safe and healthy in Wistar rats. The Mean±SD initial BW of control rats was 191.60±7.92g. As shown in Figure 1, rats’ body weights gradually increased during the experimental period. According to the result, no significant differences were detected in body weights between the control and treatment groups at any time during the research. The food consumption and water intake of all treated rats are presented in Figures 2 and 3. The experimental treatments had no influence on the food and water consumption of rats during the experiment. The results of the blood metabolites following I. tinctoria administration (14 days) are shown in Table 1.
.jpg)
In the present study, 100mg/kg I. tinctoria-treated rats showed lower ALT activities than the control rats (P<0.05). There was no difference between the other groups.
The current investigation showed no difference in serum urea concentration between treatments. Only group V (1000 mg/kg) with III (250 mg/kg) differences was close to significant (P=0.06). In the current study, 100, 250, 500, and 1000mg/kg of I. tinctoria administration had no effects on the serum HDL of rats compared to the control group. In addition, the results revealed that triglyceride concentrations in group II (100 mg/kg) were lower than in the control and groups III and IV groups (P<0.05).
According to the results, no difference in blood glucose concentration was observed between the groups receiving the extract and the control group (P>0.05). However, blood glucose concentrations in rats that received 100mg/kg BW of the extract were lower than that in group IV (P<0.05). The administration of the aqueous extract at the doses of 100, 250, 500, and 1000mg/kg led to no changes in total protein.
The histopathological analysis of rats’ testes tissue is shown in Figure 4. Animals’ testes showed normal architecture and well-organized germ cells. All treated groups revealed normal seminiferous tubules with regular arrangements of cells.
Photomicrographs from the kidney tissues of rats are presented in Figure 5. The kidney of the control group revealed a normal structure of glomeruli and proximal and distal tubules. Also, normal distal and proximal tubules were seen in all treated groups.
Histopathological observation of liver tissues from the control and extract-treated groups showed normal architecture (Figure 6). The FTIR absorption spectra between ~4000 cm-1 and ~400 cm-1 and the peak assignments of the testis, liver, and kidney tissues are shown in Figures 7-9 and presented in Tables 3-5.
.jpg)
The results of testes FTIR (Figure 7 and Table 2) indicated that three bands at ~2360 cm-1, ~696 cm-1, and ~530 cm-1 were missing in rats that received 100 mg/kg of the extract. Bands at 696 and 530 cm-1 are mainly due to nucleic acid.
.jpg)
Also, the detailed spectral analysis was done in three distinct frequency ranges, namely ~3600-3050 cm-1 (Figure 7B, 8B, and 9B), ~3050-2800 cm-1 (Figure 7C, 8C, and 9C), and ~1800-800 cm-1 (Figure 7D, 8D, and 9D) to determine the details of macromolecules changes. The peaks between ~3600 and 3050 cm-1 regions mainly consist of amide-A vibrations of proteins. The band appearing at ~3292 cm-1 in the control group did not significantly change in other treated groups (Table 2 and Figure 7B).
Figure 7C shows that the region from ~3050 to ~2800 cm-1 belongs to lipids of control and various treated groups. The bands are primarily due to CH2 asymmetric and symmetric stretching in the testis tissue. The results (Table 2 and Figure 7B) revealed that two peaks in the region of ~2926 and ~2868 cm-1 had not changed in all treated groups compared to the control.
Figure 7D shows that absorptions are due to the continuation of polysaccharides, nucleic acids, phospholipids, and carbohydrates in the range of ~1800 to ~800 cm-1. The main band at ~1235 cm-1 was because of the asymmetric stretching vibration of phospholipids, and the band observed at ~1071 cm-1 was related to PO2−asymmetric stretching: mainly nucleic acids.
According to the results presented in Tables 3 and 4, all absorption frequencies were the same in various treated groups.
.jpg)
However, the two bands at 2357 and 681 cm-1 were missing in group II kidney and liver tissues (Figure 8A, 8D, and 9A, 9B) that belong to lipids and nucleic acids. Results from Table 5 show band area ratios of amide-II/amide-I and amide-II/amide-A that indicate the protein rearrangement.

Tables 3, 4, and 5 and Figure 7A, Figure 8A, and Figure 9A show the area value of amide-I, amide-II, and amide-A, which are responsible for the secondary structure of proteins in the testis, liver, and kidney, respectively. 4. Discussion
The tendency to use natural dyes has increased worldwide [29]. The blue color of I. tinctoria is one of the oldest dyes known to humans. Indigo has an attractive blue color, high color stability, and high strength to combine with different natural colors. In the world, indigo is widely used as a medicinal plant. In Iran, the leaves of this plant are used to treat foot pain, burns, and food cracks. Also, antioxidant, anti-diabetic, anti-inflammatory, and anti-dyslipidemic properties of this plant have been reported. In the present study, oral toxicity of I. tinctoria extract was evaluated by FTIR spectroscopy combined with histopathological and serum biochemical assays in Wistar rats. Rats were divided into five groups (n=5 for each group). Group I served as the control, and the other four groups received respectively 100mg/kg (Group II), 250mg/kg (Group III), 500mg/kg (Group IV), and 1000mg of I. tinctoria per kg BW (Group V) for 14 days. The normality of data was determined using the Shapiro-Wilk normality test, all data were normal with homogen variances, and then, data were analyzed using the GLM procedure of the software.
According to the findings in the present study, the experimental treatments had no influence on the food and water consumption of rats during the experiment. Aylward et al. reported that Indigofera species did not change growth rates relative to control feeds [30]. Kumar et al. reported that animals treated with an aqueous extract of I. aspalathoides, a species of Indigofera, at a dose of 250mg/kg BW, did not alter the food and water intake [17]. Moreover, they suggested that the plant extract had no toxic side effects. A study also reported that the chemopreventive effect of I. aspalathoides and no sub-acute toxicity was observed [31]. Therefore, no alteration in BW, as well as feed and water intake of rats, may imply the nontoxic effects of I. tinctoria on these animals.
To the best of our knowledge, the toxic effect of I. tinctoria on testes, liver, and kidney, and biochemical and biophysical parameters of animal models, has not been investigated. The liver is an important body organ in metabolism and detoxification. Liver enzyme activities such as ALT and AST indicate liver injury, as enzymes leaking from liver cells cytosol into the bloodstream correlate with diseases such as diabetes, toxicant, and inflammatory processes of the liver [32, 33]. In the present study, 100mg/kg I. tinctoria-treated rats showed significant decrease of ALT level (P<0.05) with compared to control rats. There was no difference between the other groups. This finding indicates the plant hepatoprotective effect at a dose level of 100mg/kg [34]. Inconsistent with our results, Muthulingam et al. studied the impact of I. tinctoria on paracetamol-induced liver damage in rats and showed decreased ALT levels in 250 and 500mg/kg I. tinctoria-treated rats [35].
Serum creatinine and urea are the major indicators of kidney failure. The increased levels of creatinine and urea have been reported in gentamicin-induced nephrotoxicity in rats [36]. The current study showed no difference in serum urea concentration between treatments. Moreover, the serum urea concentration of 1000mg/kg and 250mg/kg of plant administration was close to significant (P=0.06). The liver also contributes to the synthesis of lipoproteins and the metabolism of cholesterol. An increase in plasma lipids, particularly cholesterol, is a common feature of diseases, such as heart disease and diabetes [37]. A study confirmed that the changes in plasma lipids could serve as a simple indicator for assessing liver disorders [38]. In the current study, I. tinctoria administration had no effects on the serum HDL of rats compared to the control group. In addition, the results revealed that triglyceride concentrations in group II (100mg/kg) were lower than in the control and groups III and IV groups (P<0.05). Tsuji et al. reported that rats’ treatment with the indigo plant (Polygonum tinctorium Lour) improved lipid profiles and suggested its application as a food supplement [39]. We found that I. tinctoria regulates lipid profiles, including TG and HDL. To our knowledge, this is the first study showing that I. tinctoria regulates lipid metabolism in vivo.
In the present study, blood glucose levels were estimated. Results indicated that 100mg/kg BW of the extract was more effective than 500mg/kg in lowering glucose concentration. Studies on the leaves of the genus Indigofera, i.e., I. tinctoria, I. affect, and I. pluchra, indicate the presence of natural products that produce hypoglycemic effects [40, 41].
Protein plays a critical role in interactions between the intra- and extra-cellular media of animal cells. Proteins are of great importance in the synthesis of microsomal detoxifying enzymes and detoxifying the toxicants that enter the body [42]. The results show that, the administration of different doses of the aqueous extract led to no changes in total protein.
The reproductive system is one of the most susceptible body organs to toxic agents. Then, the study of its response to each xenobiotic or external treatment is very important. To determine the toxic oral effect of I. tinctoria on the reproductive functions of healthy male rats, we studied the histopathological status of the testis. In the present study, no abnormalities were observed in I. tinctoria-treated rat testis. It can be explained due to the antioxidant activity of I. tinctoria.
Sreepriya et al. reported the antioxidant activity of I. tinctoria, which is very effective in oxidative stress-induced liver damage [43]. The control rats’ livers showed that the hepatic cells are radially placed, and each cell has a large spherical nucleus and granular cytoplasmic structure without any injury. Dkhil et al. reported that Indigofera oblongifolia fights against hepatic injury. The hepatoprotective effect of Indigofera can be explained due to its antioxidant activities [44]. It was shown that Indigofera oblongifolia was successful in preventing lead acetate accumulation in the liver and kidneys [45]. In the present study, biochemical results were supported by histopathological data.
The areas of the absorption bands in the FTIR spectrum are directly associated with the concentration of the molecules [46]. The results of testes FTIR showed that three bands region at ~2360 cm-1, ~696 cm-1, and ~530 cm-1 were missing in rats that received 100mg/kg of the extract. Stuart reported that a peak around ~2300 cm-1 was due to the CH-stretching of lipids [47]. Raouf et al. studied the neurons of the rat brain by using FTIR spectroscopy. They reported that the peaks between ~1000 to ~400 cm-1 regions belong to nucleic acids [48].
In the current study, according to the findings, the testes lipids and nucleic acids of group II have changed; however, no changes were observed in other I. tinctoria-treated animals. Also, the detailed spectral analysis was done in three distinct frequency ranges, to determine the details of macromolecules changes. The peaks between ~3600 and 3050 cm-1 regions mainly consist of amide-A vibrations of proteins. The band appearing at ~3292 cm-1 in the control group did not significantly change in other treated groups. This finding indicates that the protein content did not change due to various doses administered to the rats.
The main band at ~1235 cm-1 and ~1071 cm-1 were because of the asymmetric stretching vibration of phospholipids, and related to PO2− asymmetric stretching: mainly nucleic acids, respectively. Results obtained from serum lipids profiles showed that triglyceride level was decreased in 100 mg/kg of I. tinctoria administrated rats in accordance with the lipid changes in FTIR result. However, there is limited information in the literature, and further studies using FTIR are required to investigate the likely impacts of I. tinctoria extract on different body organs of model animals.
The band ratios area of amide-II/amide-I and amide-II/amide-A had not changed in any treated groups. The results show that I. tinctoria does not affect the α-helix and β-sheet secondary structure of proteins in all treated rats compared to the control group.
5. Conclusion
In recent years, the tendency to use natural colors is rising due to the side effects of some synthetic colors. Along with the growing public awareness of the dangers posed by using synthetic colors, people are returning to using natural colors that are more environmentally friendly. In the present study, oral toxicity of I. tinctoria as a source of natural color was evaluated. According to results obtained from biochemical and histopathological assays, the aqueous extract of I. tinctoria is nontoxic and safe at doses of 100, 250, 500, and 1000mg/kg BW. Moreover, FTIR findings revealed that 100mg/kg BW administration of extract caused changes at the molecular level, which requires further studies. Further investigation into its medicinal and therapeutic efficacy could also be considered.
Study limitations
The study’s major limitation is that the exact mechanism of action of I. tinctoria extract could not be explained. Therefore, further research may be needed to support our results.
Ethical Considerations
Compliance with ethical guidelines
All research procedures were approved (3818-95-4) by the Internal Committee of Animals’ Ethics and Care of the University of Jiroft. The subchronic toxicity study was done according to the OECD Guidelines No. 407.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Supervision, Writing-review, editing, and conceptualization: Abdollah Ramzani Ghara; Writing original draft, and investigation: Fereshteh Ezzati Ghadi; Investigation, data analysis, writing, and editing: Amir Mousaie.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We are thankful to the University of Jiroft for supporting this work.
References
- Carocho M, Barreiro MF, Morales P, Ferreira ICFR. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Comprehensive Reviews in Food Science and Food Safety. 2014; 13(4):377-99. [PMID]
- Modanlo H, Baghi M, Ghanbari Malidarreh A. Sunflower (Helianthus annuus L.) grain yield affected by fertilizer and plant density. Central Asian Journal of Plant Science Innovation. 2021; 1(2):102-8. [Link]
- Jain S, Nayak S, Joshi P. Hytochemical study and physical evalution of indigofera tinctoria leaves. International Journal of Comprehensive Pharmacy. 2010; 1: 1-5. [Link]
- Asadnejad S, Nabizadeh R, Nazarinia A, Jahed GR, Alimohammadi M. Data on prevalence of additive colors in local food and beverage products, Tehran, Iran Data in Brief. 2018; 19:2104-8. [PMID]
- Chung KT. Azo dyes and human health: A review. Journal of Environmental Science and Health, Part C. 2016; 34(4):233-61. [PMID]
- Silva MM, Reboredo FH, Lidon FC. Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods. 2022; 11(3):379. [PMID]
- Asuntha G, Prasannaraju Y, Prasad K. Effect of ethanol extract of Indigofera tinctoria Linn (Fabaceae) on lithium/pilocarpine-induced status epilepticus and oxidative stress in wistar rats. Tropical Journal of Pharmaceutical Research. 2010; 9(2):149-56. [DOI:10.4314/tjpr.v9i2.53702]
- Mahomoodally FM, Subratty AH, Gurib-Fakim A, Choudhary MI. Antioxidant, antiglycation and cytotoxicity evaluation of selected medicinal plants of the Mascarene Islands. BMC Complementary and Alternative Medicine. 2012; 12:165. [PMID]
- Gerometta E, Grondin I, Smadja J, Frederich M, Gauvin-Bialecki A. A review of traditional uses, phytochemistry and pharmacology of the genus Indigofera. Journal of Ethnopharmacology. 2020; 253:112608. [PMID]
- Zargaran Khouzani MR. Assessing Indigofera tinctoria L. as a forgotten medicinal-industrial plant and the importance of its revitalization for the sustainability of Iran’s agricultural ecosystems. Central Asian Journal of Environmental Science and Technology Innovation. 2022; 3(2):32-9. [DOI:10.22034/CAJESTI.2022.02.01]
- Sharma V, Agarwal A. Physicochemical and antioxidant assays of methanol and hydromethanol extract of ariel parts of Indigofera tinctoria Linn. Indian Journal of Pharmaceutical Sciences. 2015; 77(6):729-34. [PMID]
- Srinivasan S, Wankhar W, Rathinasamy S, Rajan R. Free radical scavenging potential and HPTLC analysis of Indigofera tinctoria linn (Fabaceae). Journal of Pharmaceutical Analysis. 2016; 6(2):125-31. [PMID]
- Teanglum A, Teanglum S, Saithong A. Selection of indigo plant varieties and other plants that yield indigo dye. Procedia Engineering. 2012; 32:184-90. [DOI:10.1016/j.proeng.2012.01.1255]
- Tobin BF, Feeser A, Goggin MD,. The materiality of color: The production, circulation, and application of dyes and pigments, 1400-1800. Farnham: Ashgate Publishing, Ltd; 2012. [Link]
- Degani L, Riedo C, Chiantore O. Identification of natural indigo in historical textiles by GC-MS. Analytical and Bioanalytical Chemistry. 2015; 407(6):1695-704. [PMID]
- Laitonjam WS, Wangkheirakpam SD. Comparative study of the major components of the indigo dye obtained from Strobilanthes flaccidifolius Nees. and Indigofera tinctoria Linn. International Journal of Plant Physiology and Biochemistry. 2011; 3(7):108-16. [Link]
- Kumar SS, Rao MRK, Balasubramanian MP. Antiproliferative role of Indigofera aspalathoides on 20 methylcholanthrene induced fibrosarcoma in rats. Asian Pacific Journal of Tropical Biomedicine. 2012; 2(12):966-74. [DOI:10.1016/S2221-1691(13)60008-8]
- Chen Y, Sun Y, Li W, Wei H, Long T, Li H, et al. Systems pharmacology dissection of the anti-stroke mechanism for the Chinese traditional medicine Xing-Nao-Jing. Journal of Pharmacological Sciences. 2018; 136(1):16-25. [PMID]
- Elmi A, Spina R, Abdoul-Latif F, Yagi S, Fontanay S, Risler A, et al. Rapid screening for bioactive natural compounds in Indigofera caerulea Rox fruits. Industrial Crops and Products. 2018; 125:123-30. [DOI:10.1016/j.indcrop.2018.08.089]
- Rahman TU, Zeb MA, Liaqat W, Sajid M, Hussain S, Choudhary MI. Phytochemistry and pharmacology of genus indigofera: A review. Records of Natural Products. 2018; 12(1):1-13. [DOI:10.25135/rnp.13.16.12.585]
- Gupta N, Jain U. Prominent wound healing properties of indigenous medicines. Jundishapur Journal of Natural Pharmaceutical Products. 2010; 1(1):2. [DOI:10.4103/2229-5119.73579]
- Singh R, Sharma S, Sharma V. Comparative and quantitative analysis of antioxidant and scavenging potential of Indigofera tinctoria Linn. extracts. Journal of Integrative Medicine. 2015; 13(4):269-78. [DOI:10.1016/S2095-4964(15)60183-2]
- Srinivasan S, Wankhar W, Rathinasamy S, Rajan R. Neuroprotective effects of Indigofera tinctoria on noise stress affected Wistar albino rat brain. Journal of Applied Pharmaceutical Science. 2015; 5(06):58-65. [DOI:10.7324/JAPS.2015.50609]
- Madakkannu B, Ravichandran R. In vivo immunoprotective role of Indigofera tinctoria and Scoparia dulcis aqueous extracts against chronic noise stress induced immune abnormalities in Wistar albino rats. Toxicology Reports. 2017; 4:484-93. [PMID]
- Rai V, Mukherjee R, Routray A, Ghosh AK, Roy S, Ghosh BP, et al. Serum-based diagnostic prediction of oral submucous fibrosis using FTIR spectrometry. Spectrochimica Acta Part A: Molecular Spectroscopy. 2018; 189:322-9. [PMID]
- Downes A, Mouras R, Elfick A. Optical spectroscopy for noninvasive monitoring of stem cell differentiation. Journal of Biomedicine & Biotechnology. 2010; 2010:101864. [PMID]
- Ma Y, Zhang P, Yang Y, Wang F, Qin H. Metabolomics in the fields of oncology: A review of recent research. Molecular Biology Reports. 2012; 39(7):7505-11. [PMID]
- Humason GL. Animal tissue techniques. San Francisco: Freeman; 1962. [DOI:10.5962/bhl.title.5890]
- Bechtold T, Turcanu A, Geissler S, Ganglberger E. Process balance and product quality in the production of natural indigo from Polygonum tinctorium Ait. Applying low-technology methods. Bioresour Technology. 2002; 81(3):171-7. [DOI:10.1016/S0960-8524(01)00146-8]
- Aylward JH, Haydock KP, Strickland RW, Hegarty MP. Indigofera species with agronomic potential in the tropics. Rat toxicity studies. Australian Journal of Agricultural Research. 1987; 38(1):177-86. [DOI:10.1071/AR9870177]
- Rajkapoor B, Murugesh N, Chodon D, Sakthisekaran D. Chemoprevention of N-nitrosodiethylamine induced phenobarbitol promoted liver tumors in rat by extract of Indigofera aspalathoides. Biological and Pharmaceutical Bulletin. 2005; 28(2):364-6. [DOI:10.1248/bpb.28.364] [PMID]
- Williamson EM, Okpako DT, Evans FJ. Selection, preparation and pharmacological evaluation of plant material, Volume 1. New Jersey: n Wiley; 1996. [Link]
- Music M, Dervisevic A, Pepic E, Lepara O, Fajkic A, Ascic-Buturovic B, et al. Metabolic syndrome and serum liver enzymes level at patients with type 2 diabetes mellitus. Medical Archives. 2015; 69(4):251-5. [DOI:10.5455/medarh.2015.69.251-255] [PMID] [PMCID]
- Saleem U, Amin S, Ahmad B, Azeem H, Anwar F, Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicology Reports. 2017; 4:580-5. [DOI:10.1016/j.toxrep.2017.10.005] [PMID] [PMCID]
- Muthulingam M, Mohandoss P, Indra N, Sethupathy S. Antihepatotoxic efficacy of Indigofera tinctoria (Linn.) on paracetamol induced liver damage in rats. International Journal of Pharmacy & Biomedical Research. 2010; 1(1):13-8. [Link]
- Tavafi M, Ahmadvand H, Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iranian Journal of Kidney Diseases. 2012; 6(1):25-32. [PMID]
- Rugerio-Escalona C, Ordaz-Pichardo C, Becerra-Martinez E, Cruz-López MDC, López-Y-López VE, Mendieta-Moctezuma A, et al. “Diabetes and metabolism disorders medicinal plants: A glance at the past and a look to the future 2018”: Antihyperglycemic Activity of Hamelia patens Jacq. Extracts. Evidence-Based Complementary and Alternative Medicine. 2018; 2018:7926452. [DOI:10.1155/2018/7926452] [PMID] [PMCID]
- Honma T, Suda M. Changes in plasma lipoproteins as toxicity markers for carbon tetrachloride, chloroform, and dichloromethane. Industrial Health. 1997; 35(4):519-31. [PMID]
- Tsuji H, Kondo M, Odani W, Takino T, Takeda R, Sakai T. Treatment with indigo plant (Polygonum tinctorium Lour) improves serum lipid profiles in Wistar rats fed a high-fat diet. The Journal of Medical Investigation. 2020; 67(1.2):158-62. [DOI:10.2152/jmi.67.158] [PMID]
- Bueno Pérez L, Li J, Lantvit DD, Pan L, Ninh TN, Chai H-B, et al. Bioactive constituents of Indigofera spicata. Journal of Natural Products. 2013; 76(8):1498-504. [PMID] [PMCID]
- Birru EM, Abdelwuhab M, Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complementary and Alternative Medicine. 2015; 15(1):1-8. [PMID] [PMCID]
- Hodges RE, Minich DM. Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. Journal of Nutrition and Metabolism. 2015; 2015:760689. [PMID] [PMCID]
- Sreepriya M, Devaki T, Balakrishna K, Apparanantham T. Effect of Indigofera tinctoria Linn on liver antioxidant defense system during D-galactosamine I endotoxin-induced acute hepatitis in rodents. Indian J Exp Biol. 2001; 39(2):181-4. [PMID]
- Dkhil MA, Abdel-Gaber R, Khalil MF, Hafiz TA, Mubaraki MA, Al-Shaebi EM, et al. Indigofera oblongifolia as a fight against hepatic injury caused by murine trypanosomiasis. Saudi Journal of Biological Sciences. 2020; 27(5):1390-5. [PMID] [PMCID]
- Abdel Moneim AE. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One. 2016; 11(7):e0158965. [DOI:10.1371/journal.pone.0158965] [PMID] [PMCID]
- Damian G, Cavalu S, Miclăuş V, Sabău L, Vedeanu N, Lucaciu CM. EPR and ATR-FT-IR investigation of lyophilized cytochrome C at different pH. Romanian Journal of Biophysics. 2007; 17:139-48. [Link]
- Stuart BH. Infrared spectroscopy of biological applications: An overview. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; 2006. [DOI:10.1002/9780470027318.a0208]
- Raouf GA, Qusti SY, Ali AM, Dakhakhni TH. The mechanism of 2, 4-dichlorophenoxyacetic acid neurotoxicity on rat brain tissue by using FTIR spectroscopy. Life Science Journal. 2012; 9(4):1686-97. [Link]
Type of Study: Original Article |
Subject:
Nutrition
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









.jpg)
.jpg)