Volume 12, Issue 1 (Winter 2024)
Iran J Health Sci 2024, 12(1): 27-38 |
Back to browse issues page
Ethics code: IR.TUMS.IKH.REC.1400.175
Clinical trials code: IR.TUMS.IKH.REC.1400.175
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khalili H, Emadi Koochak H, Salehi M, Dehghan Manshadi S A, Toroghi N, Nourian A, et al . Prevalence of Sputnik V COVID-19 Vaccine Side Effects Among Healthcare Workers in a Tertiary Care, Academic Center in Tehran City, Iran. Iran J Health Sci 2024; 12 (1) :27-38
URL: http://jhs.mazums.ac.ir/article-1-862-en.html
URL: http://jhs.mazums.ac.ir/article-1-862-en.html
Hossein Khalili 

 , Hamid Emadi Koochak
, Hamid Emadi Koochak 

 , Mohammadreza Salehi
, Mohammadreza Salehi 

 , Seyed Ali Dehghan Manshadi
, Seyed Ali Dehghan Manshadi 

 , Negar Toroghi
, Negar Toroghi 
 , Anahid Nourian
, Anahid Nourian 

 , Keyhan Mohammadi
, Keyhan Mohammadi 

 , Nasim Shirazi
, Nasim Shirazi 

 , Esmaeil Mohammadnejad
, Esmaeil Mohammadnejad 

 , Elnaz Shahmohamadi
, Elnaz Shahmohamadi 

 , Maliheh Hasannezhad *
, Maliheh Hasannezhad * 




 , Hamid Emadi Koochak
, Hamid Emadi Koochak 

 , Mohammadreza Salehi
, Mohammadreza Salehi 

 , Seyed Ali Dehghan Manshadi
, Seyed Ali Dehghan Manshadi 

 , Negar Toroghi
, Negar Toroghi 
 , Anahid Nourian
, Anahid Nourian 

 , Keyhan Mohammadi
, Keyhan Mohammadi 

 , Nasim Shirazi
, Nasim Shirazi 

 , Esmaeil Mohammadnejad
, Esmaeil Mohammadnejad 

 , Elnaz Shahmohamadi
, Elnaz Shahmohamadi 

 , Maliheh Hasannezhad *
, Maliheh Hasannezhad * 


Department of Infectious Diseases and Tropical Medicine, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran. , mmalihehasannezhad@yahoo.com
Full-Text [PDF 842 kb]
(551 Downloads)
| Abstract (HTML) (1697 Views)
Full-Text: (445 Views)
Introduction
Acluster of pneumonia of unknown etiology was discovered in Wuhan City, Hubei Province, China, in December 2019 [1]. Afterward, a novel coronavirus (initially named 2019-nCov) was found responsible for this unusual viral pneumonia outbreak [2]. This virus was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the disease was called coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [3]. SARSCoV2 is an enveloped positive-sense single-stranded RNA virus [4]. The virus is highly contagious and is mainly transmitted by respiratory droplets and aerosols, direct contact with mucous membranes of infected persons and surfaces, and probably the fecal-oral route [5, 6]. The initial clinical symptoms of COVID-19 are the same as other viral pneumonia, with varying degrees of severity. Typical clinical manifestations of COVID-19 are fever, dry cough, dyspnea, headache, fatigue, anorexia, myalgia, and pneumonia [2, 7]. The disease may progress to respiratory failure (owing to alveolar damage) and even death [1]. Some critically ill patients initially have mild symptoms of low-grade fever and mild cough but rapidly deteriorate. The pathophysiology involved with this rapid progress in these patients remains to be determined [8]. Complications of COVID-19 can include dysfunction of the heart, brain, lung, liver, kidney, and coagulation system. Hospital mortality from COVID-19 is about 15% to 20% [9]. There is no specific, highly effective treatment for COVID-19. Most existing treatments for COVID-19 have focused on a combination of supportive therapy, using antivirals and anti-inflammatory drugs with conflicting results [10].
Therefore, the best approach to treat COVID-19 is through proper prevention and management measures [11, 12]. Intervention modalities such as vaccinations effectively control infectious diseases, including the COVID-19 pandemic [11, 13]. The relationship between public health activity and the control of disease transmission is evident. Most countries have implemented multiple infection control measures, so it is difficult to define the relative benefit of each. The WHO recommends vaccination against SARS-CoV-2 to mitigate the COVID-19 pandemic [9, 14]. Vaccination, combined with non-pharmaceutical interventions, is the best way to control the pandemic, which comprises testing, contract tracing, and up-to-date anti-COVID-19 treatments [15, 16].
Several approved and candidate vaccines for COVID-19 have been developed using different platforms [17]. Sputnik V is a COVID-19 vaccine, which is a viral two-dose vector vaccine based on two human adenoviruses. It is administered in two doses, separated by at least 21 days. The first component contains a recombinant adenovirus type 26 vector, and the second dose contains a recombinant adenovirus type 5 vector. Both vectors carry the gene for the full-length SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S) [14]. The Russian Ministry of Health registered it as Gam-COVID-Vac in August 2020 and starting from December 2020, it will be mass-distributed in several countries. The effectiveness of the Sputnik V vaccine against COVID-19 has been announced to be 91.6%. However, it may be less effective in the elderly or patients with immunodeficiency [18, 19]. In this study, local and systemic flu-like reactions were more common in the vaccine group, and no serious adverse events were deemed related to the vaccine. The safety and high tolerability profile of the Sputnik V vaccine were demonstrated in the preliminary and interim results of the ongoing ROCCA study [20].
The information about possible vaccine-related adverse effects in the actual world among healthcare providers is still imprecise. Evidence from clinical trials preceding approval is necessary but not adequate to determine the safety of vaccines. The safety should be evaluated in a real-world setting, focusing. Therefore, this study’s primary objective was to estimate the prevalence of Sputnik V COVID-19 vaccine-associated side effects among healthcare workers in a teaching hospital in Tehran City, Iran. The results of this study will be reassuring to those who are fearful of the Sputnik V COVID-19 vaccine. So, this study aimed to provide evidence on the side effects of the Sputnik V COVID-19 vaccine among female and male healthcare providers after receiving the first and second doses.
2. Materials and Methods
Study design, setting, and participants
This survey was considered a cohort retrospective study from March 2021 to August 2021. It aimed to evaluate the prevalence of the various side effects following the completion of the Sputnik V dose schedule (had received both the first and second dose) among healthcare workers of tertiary referral care, academic medical center hospital, Imam Khomeini Hospital Complex affiliated to the Tehran University of Medical Sciences. All the healthcare workers of the hospital who had received the COVID-19 vaccination within the specified period were included in this study if they consented.
After initial approval of the study and obtaining consent form, each participant’s demographic and clinical characteristics were received. No financial incentives or compensations were given to the participants throughout the study. The participants who declined consent were not permitted to enroll in the survey. The participants could withdraw from the study at any time in line with stipulations of the World Medical Association (WMA) Declaration of the Helsinki ethical principles [21].
The study data were gathered via phone calls or interviews during the first 3 months after vaccination. The study questionnaire was developed based on the WHO’s “causality assessment of an adverse event following immunization” manual [22]. The data of each person were collected via the questionnaire of multiple-choice items in which all required information was included. The questionnaire included three sections. In the first section, demographic questions such as gender, age, education level, and employment were included. The second section reviewed the participant’s chronic conditions and medical/drug/social/habitual history, such as the history of any specific disease (i.e. diabetes, hypertension, cardiovascular diseases, cancer, autoimmune diseases, and chronic respiratory diseases), history of documented COVID-19 infection (positive PCR or involvement of lung), history of other vaccinations during the previous year of vaccination and history of allergic reactions to vaccination. The last section related the rate, severity, and other characteristics of vaccine-related side effects after each dose, such as local, allergic, and organ-specific side effects. This survey was designed based on previous extensive literature search studies on the expected post-COVID-19 vaccine adverse effects. It was validated by a group of experts who provided feedback on the different items of the survey. The survey questions were multiple choice and were created in Persian and English.
Statistical analysis
All statistical tests were executed using the SPSS software, version 24 (SPSS Inc. Chicago, IL, USA). Primarily, descriptive statistics were performed on the demographic variables, medical history, COVID-19-related history, and other baseline variables. These data and the rate of vaccine side effects after each dose were represented by frequencies, percentages, Mean±SD. Associations between adverse effects of the COVID-19 vaccine reported by participants’ gender of the included population (female vs male) and medical history and COVID-19 history were investigated using the chi-square test. P<0.05 was considered statistically significant at a 95% confidence interval. Ordinal regression was used to identify various factors that could predict the severity of adverse effects after the first and second Sputnik V vaccines. The logit model was used for this purpose.
Results
Demographic characteristics
A total of 372 participants with eligible criteria filled in the questionnaire properly during the study period and were included in the final analyses. The Mean±SD age of all participants was 36.5±9.18 years. Also, 202 participants (54.3%) were female. The Mean±SD ages of female and male subjects were 34.91±8.32 and 38.38±9.18 years, respectively. Table 1 presents the baseline demographic characteristics of the study population.
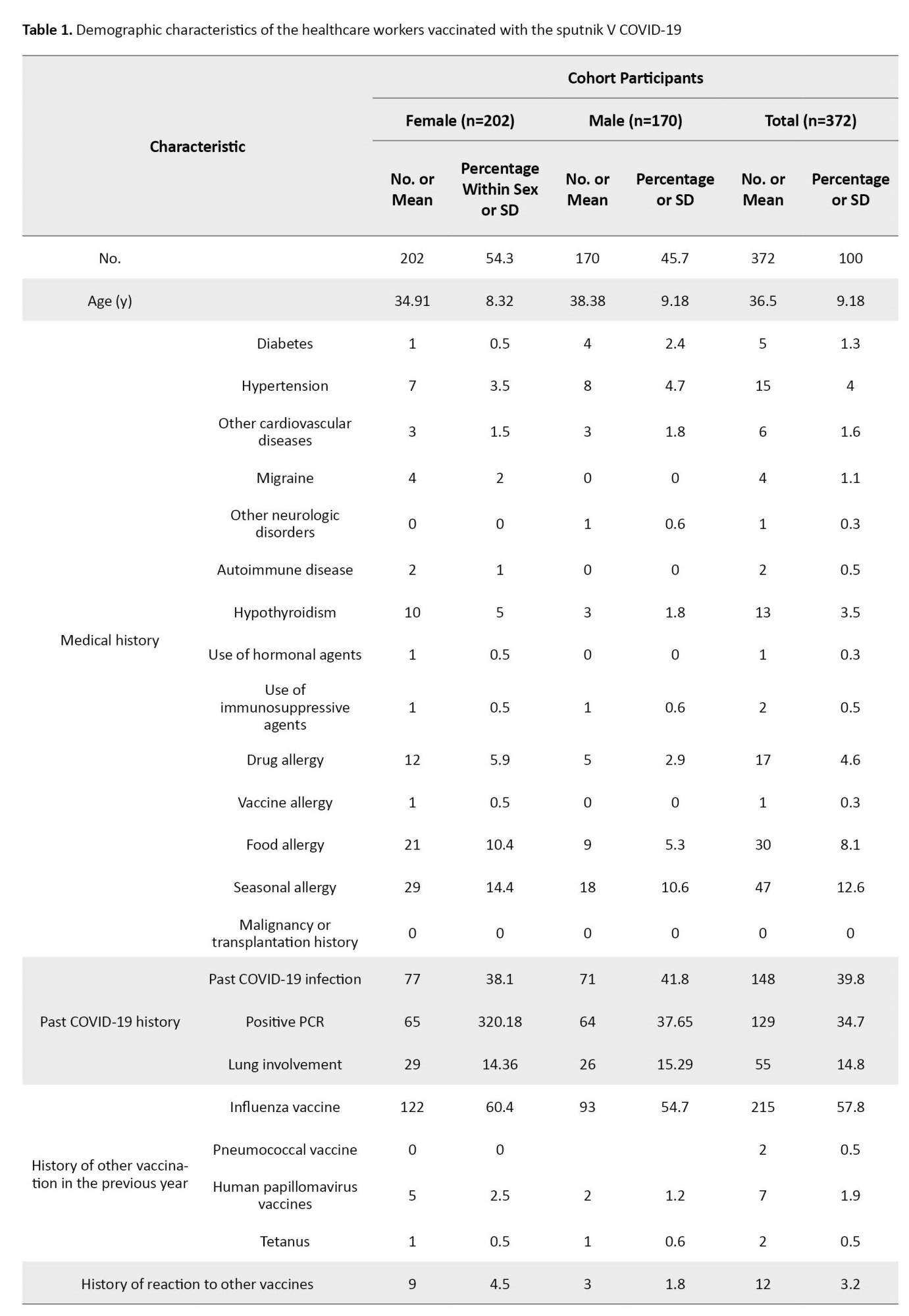
The most prevalent chronic medical conditions were hypertension (15%) and hypothyroidism (13%), as shown in Table 1.
As shown in Table 1, 148 participants (39.8%) had been previously infected by COVID-19 (confirmed by positive PCR or history of lung involvement) (77 females and 71 males). The documented positive PCR was noted among 129 participants (65 in the female and 64 in the male group). Fifty-five patients (29 females and 26 males) had a history of lung involvement due to COVID-19 infection. Also, 215(57.8%) had a history of influenza vaccination during the previous year. Only 12 (3.2%) reported a history of considerable adverse reactions to other vaccines.
Reported side effects after the first dose of Sputnik V COVID-19 vaccine
The prevalence of various adverse reactions to the first dose of the Sputnik V COVID-19 vaccine in participants (female vs male) was presented in Table 2.

A total of 287 participants (77.15%) (172 in the female group and 115 in the male group) reported having at least one side effect following the first dose of the COVID-19 vaccine. In addition, 30 females and 55 males did not experience any side effects. All reported side effects were considered grade 1 or 2, and no severe cases requiring hospitalization or leading to permanent dysfunction or death were noted. The most common side effects were myalgia (180; 48.4%), followed by injection site pain (98; 26.3%), fever (73; 19.6%), headache (62; 16.7%), and fatigue (47; 12.6%). The most frequent side effects following the first dose administration were more prevalent in the female group than the male group, with a statistically significant difference in the case of fever (26.7% vs 11.2%, P<0.001), injection site pain (33.7% vs 17.6%, P<0.001), fever (26.7% vs 11.2%, P<0.001), myalgia (58.9% vs 35.9%; P<0.001), arthralgia (7.4% vs 2.4%, P<0.027) and diarrhea (6.9% vs 1.8%, P=0.017). As shown in Table 3, the most reported side effects among the male participants were grade 1 (82 cases, 71% of adverse effects) rather than grade 2 (33 cases, 29% of adverse effects), and no medical treatment was needed.
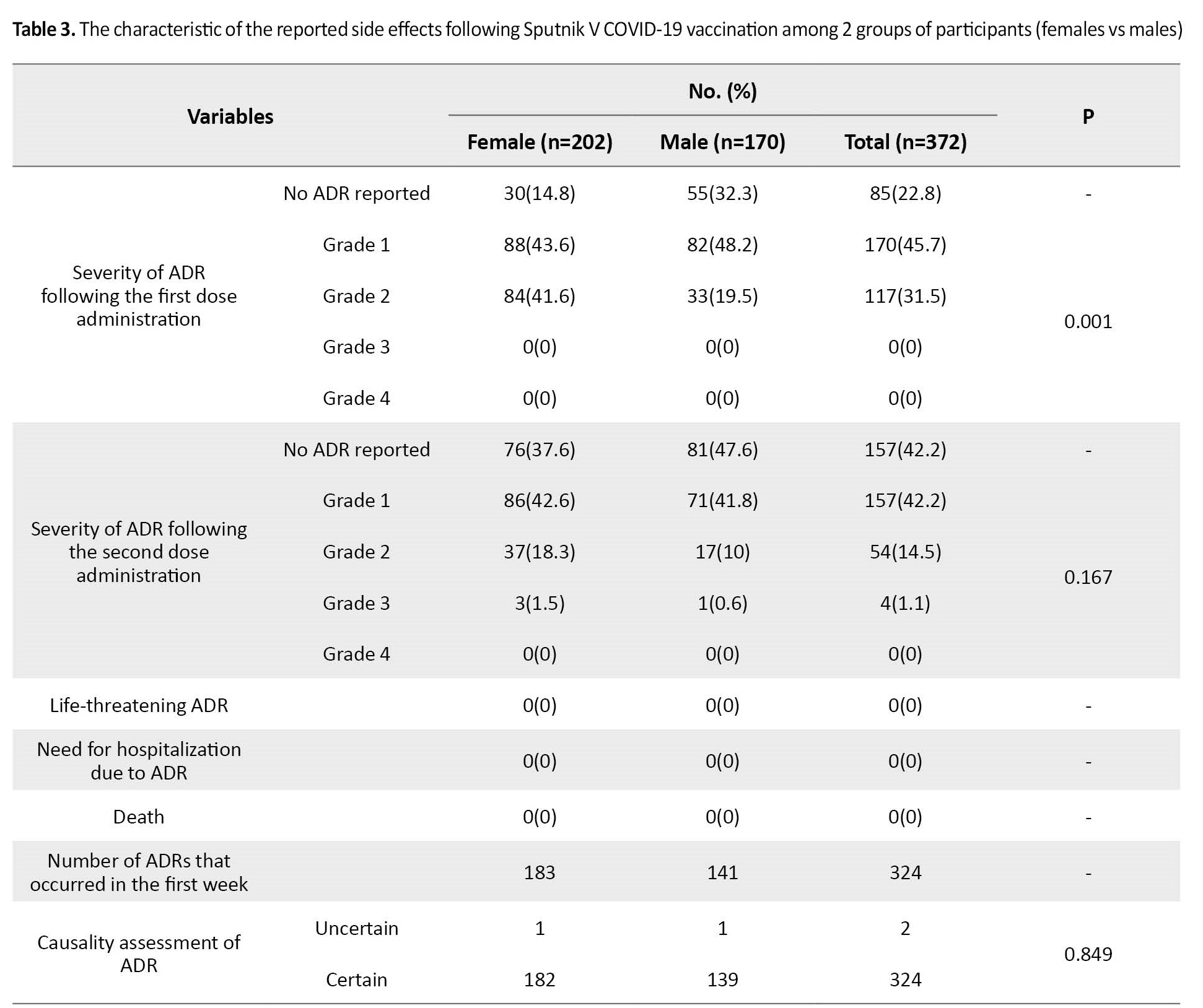
However, in the female group, the rate of grade 2 adverse effects (84 cases, 49%) was approximately similar to the rate of grade 1 adverse effects (88 cases, 51%).
Reported side effects after the second dose of Sputnik V COVID-19 vaccine
Table 4 presents various side effects following the second Sputnik V COVID-19 vaccine dose.

The overall number of cohort participants that reported at least one side effect after administering the second dose of vaccine is 215 (57.8%; 126 in female and 89 in male participants). No side effects were noted in 157 patients (76 female and 81 male cases). Three and one cases of grade 3 side effects were reported in the female and male cohorts, respectively. No case of grade 4 and life-threatening side effects or death was seen. The most common side effects among participants were myalgia (28.2%), fever (12.9%), injection site pain (12.6%), and headache (11.8%). The characteristics of the reported side effects are shown in Table 3. Totally, the rate of most side effects in the female group was higher than in the male group. However, this difference was not statistically significant. The most reported side effects in the female patients were grade 1 (86 cases, 68.2% of side effects), then grade 2 (37 cases, 29.4%) and grade 3 (3 cases, 2.4%). Among the male cohort, the rate of grade 1 side effects (71 cases; 79.8%) was higher than grade 2 (17 cases; 19.1%) and grade 3 (one case; 1.1%).
Association between COVID-19 vaccine side effects and medical history
The association between the composite variables of side effects and past medical conditions was investigated (Table 5).
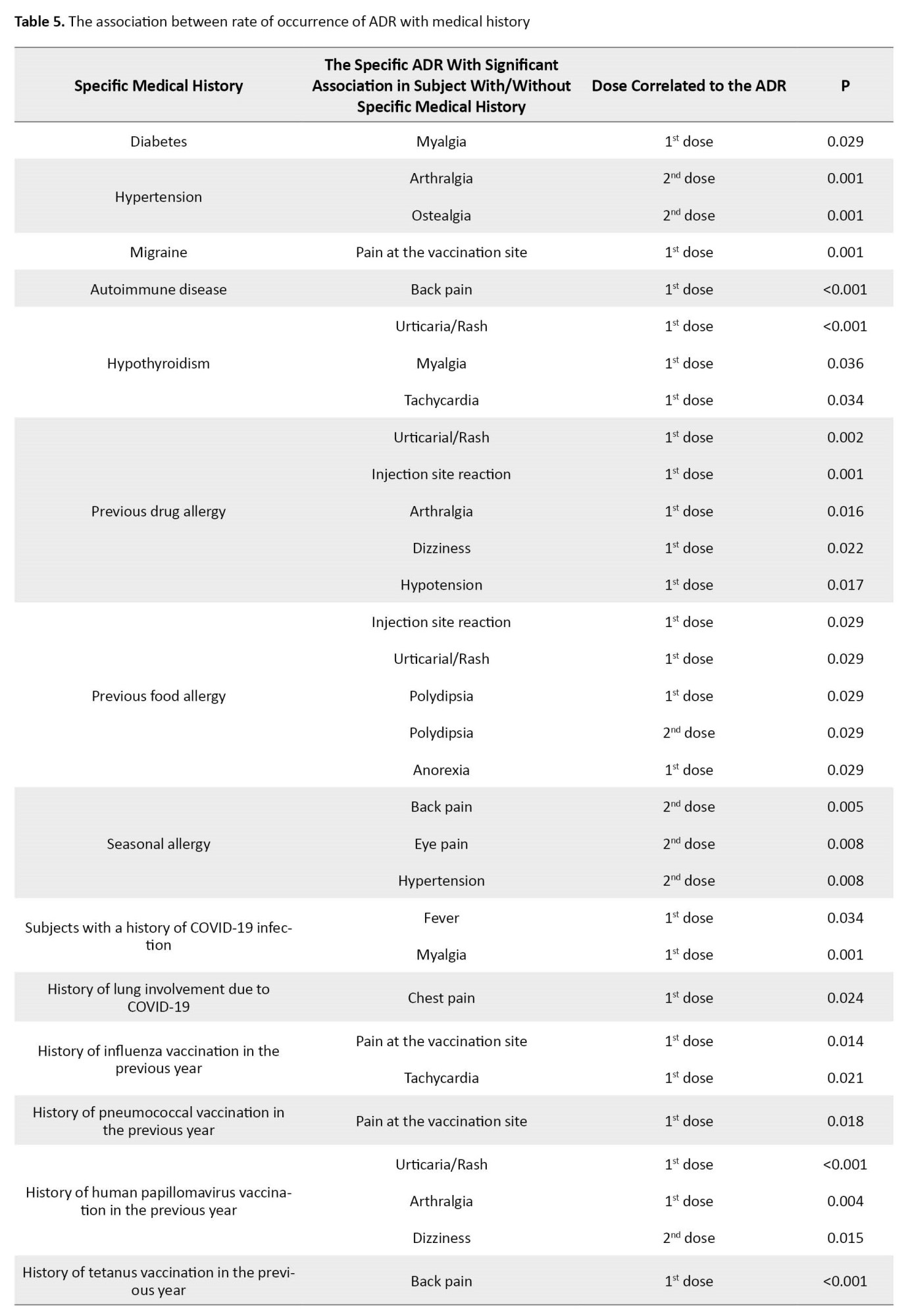
Interestingly, among the subjects with a history of COVID-19, the rate of fever after the first dose (P=0.034) and myalgia after the first dose (P=0.001) was higher than subjects without a history of COVID-19. A higher rate of chest pain after the first dose (P=0.024) was seen in cases with a history of lung involvement. Among patients with a history of influenza vaccination in the previous year, the rate of pain at the vaccination site after the first dose (P=0.014) and tachycardia after the first dose (P=0.021) was higher. A higher rate of pain was seen following the first vaccination dose in subjects with a previous history of pneumococcal vaccination (P=0.018). The last vaccination with HPV was associated with a higher rate of urticaria/rash (P<0.001) and arthralgia (P=0.004) after the first dose and dizziness (P=0.015) after the second dose.
Among the examined variables, the sex of the subjects (estimate=-0.923, P=0.001) and the history of COVID-19 infection (estimate=0.816, P=0.002) had a significant association with predicting the severity of the adverse effects after the first dose in this model. To predict the severity of adverse effects after the second dose, the subjects’ sex (estimate=-1.34, P=0.010) and status of hypertension history (estimate=2.075, P=0.039) were considered significant.
Discussion
The present study reports the possible adverse reactions after receiving Sputnik V COVID-19 vaccination. In this study, at least one side effect with the Sputnik V vaccine was reported at 77.15% following the first dose and 57.8% following the second dose among the 372 vaccinated healthcare workers. The most common side effects following the first dose were myalgia, injection site pain, fever, and headache. Regarding the association between participants’ sex and reported side effects, the post-first dose reported side effects were more prevalent in the female group. In contrast, the association between sex and side effects was not significant after the second dose. The most common dose side effects following the second dose were myalgia, fever, injection site pain, and headache. The severity of most adverse reactions was mild to moderate (grades 1 and 2). No serious case requiring hospitalization was reported. Three and one cases of grade 3 side effects were reported after the second dose in the female and male cohorts, respectively. No case of grade 4 and life-threatening side effects or death was seen. Most of the post-first-dose administration side effects were more and reported side effects.
These results are almost identical to the data obtained from the phase 1/2 studies [19] and phase 3 [18] Sputnik V vaccines. In a non-randomized phase 1/2 studies from Russia, the most common systemic and local reactions were pain at the injection site, hyperthermia, headache, asthenia, and muscle and joint pain. Most systemic and local reactions were mild. No serious adverse events were reported, and all participants were clinically well throughout the study. In a randomized controlled phase 3 trial in Russia, which recruited 21977 volunteers, the most common adverse events were flu-like illness, injection site reactions, headache, and asthenia. Also, most of the reported adverse events were grade 1. Also, 451(5.66%) were grade 2, and 30 (0.38%) were grade 3. In this study, no serious adverse events were associated with vaccination.
The results of the ROCCA study [20] that evaluated the safety of the Sputnik V vaccine of 2558 vaccine recipients in the Republic of San Marino using active surveillance were close to our study. Hypertension was the most frequent coexisting condition, similar to our result. In this study’s interim analysis and early result, the main symptoms were local pain, asthenia, headache, and arthralgia. However, the rate of adverse effects following the second dose (66.8%) was more than the first dose (53.3%), contrary to our study, in which the rate of side effects was higher after the first dose. This difference in result may be due to various factors that could affect the rate of reported side effects, such as the status of the included population (healthcare providers vs overall population) or the age of the participants (young vs older). No serious adverse events and no deaths were reported, and about all reported AEFI (adverse event following immunization) were mild or moderate and or lasted less than 48 hours, and in more than two-thirds of cases, did not need any medication. Overall, in agreement with the result of our studies, this analysis suggests that Sputnik V has a high tolerability profile regarding short-term side effects in the population aged ≥60 years.
As mentioned previously, most reported side effects following the first dose administration, such as fever, injection site pain, fever, myalgia, arthralgia, and diarrhea, were more prevalent in the female group. Similar to this result, a study in Buenos Aires and Argentina on the incidence of early events supposedly attributable to vaccination or immunization that occurred in 707 healthcare workers [14] reported a higher rate of side effects in females. This study’s most reported side effects were muscle pain, local reactions (including pain, redness, and swelling at the injection site), fever, and diarrhea. In this study, 5% had serious adverse events, and one participant had to be hospitalized, inconsistent with our observation. Our study investigated the association between the rate of various side effects and past medical conditions. The rates of arthralgia and ostealgia after the second dose were significantly higher in participants with a history of hypertension. In addition, a higher rate of urticaria/rash, myalgia, and tachycardia was seen after the first dose among hypothyroid cases. The participants with a history of migraine experienced a higher rate of pain at the injection site.
Interestingly, a significant association was detected between a history of drug allergy and the rate of urticaria/rash, injection site reaction, arthralgia, dizziness, and hypotension after the first dose. The relationship between the history of COVID-19 infection and the rate of side effects was also investigated. Remarkably, the rate of fever and myalgia after the first dose was higher in subjects with a history of COVID-19, and the rate of chest pain after the first dose was higher if the history was positive for lung involvement due to COVID-19. The association between adverse reactions following the COVID-19 vaccine and the previous infection of SARS-CoV-2 was also seen in several studies [23, 24, 25, 26]. The sex of the person and history of COVID-19 infection and hypertension could help us to predict the severity of side effects after COVID-19 vaccination based on our results.
Conclusion
These data generally show that the heterologous vaccine based on rAd26-S and rAd5-S is safe, well-tolerated, and does not cause serious adverse events in a healthy adult. However, limitations of our study, including the low number of participants, a specific population of subjects (health care providers), and a limited age range of participants, should be considered. Further research is needed to evaluate the safety of various vaccines in diverse and larger populations with variable demographic and social characteristics.
Study strengths and limitations
The main strength of our study was the slight chance of non-response bias, as it was an interview-based study among the vaccinated healthcare population of the referral hospital. In addition, this study evaluated the various local and systemic side effects after both doses. Various confounders that could affect the rate of side effects were also investigated. The findings of this study could provide useful insight concerning the high tolerability profile of the COVID-19 vaccine and may play an essential role in reducing vaccine hesitancy among the public. The sample size of our study was considered a significant limitation. The safety profile of the Sputnik V vaccine compared to other vaccines and among other populations should be evaluated in further studies with larger sample sizes across the country’s different regions.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted in accordance with the principles stated in the Declaration of Helsinki. All participants who completed the questionnaires were requested to provide written informed consent as a requirement for their participation in this study. The research protocol and written consent forms were reviewed and approved by the Ethics Committee of the Tehran University of Medical Sciences (Code: IR.TUMS.IKHC.REC.1400.175).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and supervision: Maliheh Hasannezhad, Hossein Khalili and Hamid Emadi Koochak; Methodology: Maliheh Hasannezhad and Mohammad Reza Salehi; Data analysis: Hossein Khalili and Esmaeil Mohammadnejad; Investigation and writing the original draft: Seyed Ali Dehghan Manshadi, Negar Toroghi, Anahid Nourian, Keyhan Mohammadi and Nasim Shirazi; Review and editing: Maliheh Hasannezhad and Elnaz Shahmohamadi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Imam Khomeini Hospital Complex staff for their help.
References
Acluster of pneumonia of unknown etiology was discovered in Wuhan City, Hubei Province, China, in December 2019 [1]. Afterward, a novel coronavirus (initially named 2019-nCov) was found responsible for this unusual viral pneumonia outbreak [2]. This virus was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the disease was called coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [3]. SARSCoV2 is an enveloped positive-sense single-stranded RNA virus [4]. The virus is highly contagious and is mainly transmitted by respiratory droplets and aerosols, direct contact with mucous membranes of infected persons and surfaces, and probably the fecal-oral route [5, 6]. The initial clinical symptoms of COVID-19 are the same as other viral pneumonia, with varying degrees of severity. Typical clinical manifestations of COVID-19 are fever, dry cough, dyspnea, headache, fatigue, anorexia, myalgia, and pneumonia [2, 7]. The disease may progress to respiratory failure (owing to alveolar damage) and even death [1]. Some critically ill patients initially have mild symptoms of low-grade fever and mild cough but rapidly deteriorate. The pathophysiology involved with this rapid progress in these patients remains to be determined [8]. Complications of COVID-19 can include dysfunction of the heart, brain, lung, liver, kidney, and coagulation system. Hospital mortality from COVID-19 is about 15% to 20% [9]. There is no specific, highly effective treatment for COVID-19. Most existing treatments for COVID-19 have focused on a combination of supportive therapy, using antivirals and anti-inflammatory drugs with conflicting results [10].
Therefore, the best approach to treat COVID-19 is through proper prevention and management measures [11, 12]. Intervention modalities such as vaccinations effectively control infectious diseases, including the COVID-19 pandemic [11, 13]. The relationship between public health activity and the control of disease transmission is evident. Most countries have implemented multiple infection control measures, so it is difficult to define the relative benefit of each. The WHO recommends vaccination against SARS-CoV-2 to mitigate the COVID-19 pandemic [9, 14]. Vaccination, combined with non-pharmaceutical interventions, is the best way to control the pandemic, which comprises testing, contract tracing, and up-to-date anti-COVID-19 treatments [15, 16].
Several approved and candidate vaccines for COVID-19 have been developed using different platforms [17]. Sputnik V is a COVID-19 vaccine, which is a viral two-dose vector vaccine based on two human adenoviruses. It is administered in two doses, separated by at least 21 days. The first component contains a recombinant adenovirus type 26 vector, and the second dose contains a recombinant adenovirus type 5 vector. Both vectors carry the gene for the full-length SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S) [14]. The Russian Ministry of Health registered it as Gam-COVID-Vac in August 2020 and starting from December 2020, it will be mass-distributed in several countries. The effectiveness of the Sputnik V vaccine against COVID-19 has been announced to be 91.6%. However, it may be less effective in the elderly or patients with immunodeficiency [18, 19]. In this study, local and systemic flu-like reactions were more common in the vaccine group, and no serious adverse events were deemed related to the vaccine. The safety and high tolerability profile of the Sputnik V vaccine were demonstrated in the preliminary and interim results of the ongoing ROCCA study [20].
The information about possible vaccine-related adverse effects in the actual world among healthcare providers is still imprecise. Evidence from clinical trials preceding approval is necessary but not adequate to determine the safety of vaccines. The safety should be evaluated in a real-world setting, focusing. Therefore, this study’s primary objective was to estimate the prevalence of Sputnik V COVID-19 vaccine-associated side effects among healthcare workers in a teaching hospital in Tehran City, Iran. The results of this study will be reassuring to those who are fearful of the Sputnik V COVID-19 vaccine. So, this study aimed to provide evidence on the side effects of the Sputnik V COVID-19 vaccine among female and male healthcare providers after receiving the first and second doses.
2. Materials and Methods
Study design, setting, and participants
This survey was considered a cohort retrospective study from March 2021 to August 2021. It aimed to evaluate the prevalence of the various side effects following the completion of the Sputnik V dose schedule (had received both the first and second dose) among healthcare workers of tertiary referral care, academic medical center hospital, Imam Khomeini Hospital Complex affiliated to the Tehran University of Medical Sciences. All the healthcare workers of the hospital who had received the COVID-19 vaccination within the specified period were included in this study if they consented.
After initial approval of the study and obtaining consent form, each participant’s demographic and clinical characteristics were received. No financial incentives or compensations were given to the participants throughout the study. The participants who declined consent were not permitted to enroll in the survey. The participants could withdraw from the study at any time in line with stipulations of the World Medical Association (WMA) Declaration of the Helsinki ethical principles [21].
The study data were gathered via phone calls or interviews during the first 3 months after vaccination. The study questionnaire was developed based on the WHO’s “causality assessment of an adverse event following immunization” manual [22]. The data of each person were collected via the questionnaire of multiple-choice items in which all required information was included. The questionnaire included three sections. In the first section, demographic questions such as gender, age, education level, and employment were included. The second section reviewed the participant’s chronic conditions and medical/drug/social/habitual history, such as the history of any specific disease (i.e. diabetes, hypertension, cardiovascular diseases, cancer, autoimmune diseases, and chronic respiratory diseases), history of documented COVID-19 infection (positive PCR or involvement of lung), history of other vaccinations during the previous year of vaccination and history of allergic reactions to vaccination. The last section related the rate, severity, and other characteristics of vaccine-related side effects after each dose, such as local, allergic, and organ-specific side effects. This survey was designed based on previous extensive literature search studies on the expected post-COVID-19 vaccine adverse effects. It was validated by a group of experts who provided feedback on the different items of the survey. The survey questions were multiple choice and were created in Persian and English.
Statistical analysis
All statistical tests were executed using the SPSS software, version 24 (SPSS Inc. Chicago, IL, USA). Primarily, descriptive statistics were performed on the demographic variables, medical history, COVID-19-related history, and other baseline variables. These data and the rate of vaccine side effects after each dose were represented by frequencies, percentages, Mean±SD. Associations between adverse effects of the COVID-19 vaccine reported by participants’ gender of the included population (female vs male) and medical history and COVID-19 history were investigated using the chi-square test. P<0.05 was considered statistically significant at a 95% confidence interval. Ordinal regression was used to identify various factors that could predict the severity of adverse effects after the first and second Sputnik V vaccines. The logit model was used for this purpose.
Results
Demographic characteristics
A total of 372 participants with eligible criteria filled in the questionnaire properly during the study period and were included in the final analyses. The Mean±SD age of all participants was 36.5±9.18 years. Also, 202 participants (54.3%) were female. The Mean±SD ages of female and male subjects were 34.91±8.32 and 38.38±9.18 years, respectively. Table 1 presents the baseline demographic characteristics of the study population.

The most prevalent chronic medical conditions were hypertension (15%) and hypothyroidism (13%), as shown in Table 1.
As shown in Table 1, 148 participants (39.8%) had been previously infected by COVID-19 (confirmed by positive PCR or history of lung involvement) (77 females and 71 males). The documented positive PCR was noted among 129 participants (65 in the female and 64 in the male group). Fifty-five patients (29 females and 26 males) had a history of lung involvement due to COVID-19 infection. Also, 215(57.8%) had a history of influenza vaccination during the previous year. Only 12 (3.2%) reported a history of considerable adverse reactions to other vaccines.
Reported side effects after the first dose of Sputnik V COVID-19 vaccine
The prevalence of various adverse reactions to the first dose of the Sputnik V COVID-19 vaccine in participants (female vs male) was presented in Table 2.

A total of 287 participants (77.15%) (172 in the female group and 115 in the male group) reported having at least one side effect following the first dose of the COVID-19 vaccine. In addition, 30 females and 55 males did not experience any side effects. All reported side effects were considered grade 1 or 2, and no severe cases requiring hospitalization or leading to permanent dysfunction or death were noted. The most common side effects were myalgia (180; 48.4%), followed by injection site pain (98; 26.3%), fever (73; 19.6%), headache (62; 16.7%), and fatigue (47; 12.6%). The most frequent side effects following the first dose administration were more prevalent in the female group than the male group, with a statistically significant difference in the case of fever (26.7% vs 11.2%, P<0.001), injection site pain (33.7% vs 17.6%, P<0.001), fever (26.7% vs 11.2%, P<0.001), myalgia (58.9% vs 35.9%; P<0.001), arthralgia (7.4% vs 2.4%, P<0.027) and diarrhea (6.9% vs 1.8%, P=0.017). As shown in Table 3, the most reported side effects among the male participants were grade 1 (82 cases, 71% of adverse effects) rather than grade 2 (33 cases, 29% of adverse effects), and no medical treatment was needed.

However, in the female group, the rate of grade 2 adverse effects (84 cases, 49%) was approximately similar to the rate of grade 1 adverse effects (88 cases, 51%).
Reported side effects after the second dose of Sputnik V COVID-19 vaccine
Table 4 presents various side effects following the second Sputnik V COVID-19 vaccine dose.

The overall number of cohort participants that reported at least one side effect after administering the second dose of vaccine is 215 (57.8%; 126 in female and 89 in male participants). No side effects were noted in 157 patients (76 female and 81 male cases). Three and one cases of grade 3 side effects were reported in the female and male cohorts, respectively. No case of grade 4 and life-threatening side effects or death was seen. The most common side effects among participants were myalgia (28.2%), fever (12.9%), injection site pain (12.6%), and headache (11.8%). The characteristics of the reported side effects are shown in Table 3. Totally, the rate of most side effects in the female group was higher than in the male group. However, this difference was not statistically significant. The most reported side effects in the female patients were grade 1 (86 cases, 68.2% of side effects), then grade 2 (37 cases, 29.4%) and grade 3 (3 cases, 2.4%). Among the male cohort, the rate of grade 1 side effects (71 cases; 79.8%) was higher than grade 2 (17 cases; 19.1%) and grade 3 (one case; 1.1%).
Association between COVID-19 vaccine side effects and medical history
The association between the composite variables of side effects and past medical conditions was investigated (Table 5).

Interestingly, among the subjects with a history of COVID-19, the rate of fever after the first dose (P=0.034) and myalgia after the first dose (P=0.001) was higher than subjects without a history of COVID-19. A higher rate of chest pain after the first dose (P=0.024) was seen in cases with a history of lung involvement. Among patients with a history of influenza vaccination in the previous year, the rate of pain at the vaccination site after the first dose (P=0.014) and tachycardia after the first dose (P=0.021) was higher. A higher rate of pain was seen following the first vaccination dose in subjects with a previous history of pneumococcal vaccination (P=0.018). The last vaccination with HPV was associated with a higher rate of urticaria/rash (P<0.001) and arthralgia (P=0.004) after the first dose and dizziness (P=0.015) after the second dose.
Among the examined variables, the sex of the subjects (estimate=-0.923, P=0.001) and the history of COVID-19 infection (estimate=0.816, P=0.002) had a significant association with predicting the severity of the adverse effects after the first dose in this model. To predict the severity of adverse effects after the second dose, the subjects’ sex (estimate=-1.34, P=0.010) and status of hypertension history (estimate=2.075, P=0.039) were considered significant.
Discussion
The present study reports the possible adverse reactions after receiving Sputnik V COVID-19 vaccination. In this study, at least one side effect with the Sputnik V vaccine was reported at 77.15% following the first dose and 57.8% following the second dose among the 372 vaccinated healthcare workers. The most common side effects following the first dose were myalgia, injection site pain, fever, and headache. Regarding the association between participants’ sex and reported side effects, the post-first dose reported side effects were more prevalent in the female group. In contrast, the association between sex and side effects was not significant after the second dose. The most common dose side effects following the second dose were myalgia, fever, injection site pain, and headache. The severity of most adverse reactions was mild to moderate (grades 1 and 2). No serious case requiring hospitalization was reported. Three and one cases of grade 3 side effects were reported after the second dose in the female and male cohorts, respectively. No case of grade 4 and life-threatening side effects or death was seen. Most of the post-first-dose administration side effects were more and reported side effects.
These results are almost identical to the data obtained from the phase 1/2 studies [19] and phase 3 [18] Sputnik V vaccines. In a non-randomized phase 1/2 studies from Russia, the most common systemic and local reactions were pain at the injection site, hyperthermia, headache, asthenia, and muscle and joint pain. Most systemic and local reactions were mild. No serious adverse events were reported, and all participants were clinically well throughout the study. In a randomized controlled phase 3 trial in Russia, which recruited 21977 volunteers, the most common adverse events were flu-like illness, injection site reactions, headache, and asthenia. Also, most of the reported adverse events were grade 1. Also, 451(5.66%) were grade 2, and 30 (0.38%) were grade 3. In this study, no serious adverse events were associated with vaccination.
The results of the ROCCA study [20] that evaluated the safety of the Sputnik V vaccine of 2558 vaccine recipients in the Republic of San Marino using active surveillance were close to our study. Hypertension was the most frequent coexisting condition, similar to our result. In this study’s interim analysis and early result, the main symptoms were local pain, asthenia, headache, and arthralgia. However, the rate of adverse effects following the second dose (66.8%) was more than the first dose (53.3%), contrary to our study, in which the rate of side effects was higher after the first dose. This difference in result may be due to various factors that could affect the rate of reported side effects, such as the status of the included population (healthcare providers vs overall population) or the age of the participants (young vs older). No serious adverse events and no deaths were reported, and about all reported AEFI (adverse event following immunization) were mild or moderate and or lasted less than 48 hours, and in more than two-thirds of cases, did not need any medication. Overall, in agreement with the result of our studies, this analysis suggests that Sputnik V has a high tolerability profile regarding short-term side effects in the population aged ≥60 years.
As mentioned previously, most reported side effects following the first dose administration, such as fever, injection site pain, fever, myalgia, arthralgia, and diarrhea, were more prevalent in the female group. Similar to this result, a study in Buenos Aires and Argentina on the incidence of early events supposedly attributable to vaccination or immunization that occurred in 707 healthcare workers [14] reported a higher rate of side effects in females. This study’s most reported side effects were muscle pain, local reactions (including pain, redness, and swelling at the injection site), fever, and diarrhea. In this study, 5% had serious adverse events, and one participant had to be hospitalized, inconsistent with our observation. Our study investigated the association between the rate of various side effects and past medical conditions. The rates of arthralgia and ostealgia after the second dose were significantly higher in participants with a history of hypertension. In addition, a higher rate of urticaria/rash, myalgia, and tachycardia was seen after the first dose among hypothyroid cases. The participants with a history of migraine experienced a higher rate of pain at the injection site.
Interestingly, a significant association was detected between a history of drug allergy and the rate of urticaria/rash, injection site reaction, arthralgia, dizziness, and hypotension after the first dose. The relationship between the history of COVID-19 infection and the rate of side effects was also investigated. Remarkably, the rate of fever and myalgia after the first dose was higher in subjects with a history of COVID-19, and the rate of chest pain after the first dose was higher if the history was positive for lung involvement due to COVID-19. The association between adverse reactions following the COVID-19 vaccine and the previous infection of SARS-CoV-2 was also seen in several studies [23, 24, 25, 26]. The sex of the person and history of COVID-19 infection and hypertension could help us to predict the severity of side effects after COVID-19 vaccination based on our results.
Conclusion
These data generally show that the heterologous vaccine based on rAd26-S and rAd5-S is safe, well-tolerated, and does not cause serious adverse events in a healthy adult. However, limitations of our study, including the low number of participants, a specific population of subjects (health care providers), and a limited age range of participants, should be considered. Further research is needed to evaluate the safety of various vaccines in diverse and larger populations with variable demographic and social characteristics.
Study strengths and limitations
The main strength of our study was the slight chance of non-response bias, as it was an interview-based study among the vaccinated healthcare population of the referral hospital. In addition, this study evaluated the various local and systemic side effects after both doses. Various confounders that could affect the rate of side effects were also investigated. The findings of this study could provide useful insight concerning the high tolerability profile of the COVID-19 vaccine and may play an essential role in reducing vaccine hesitancy among the public. The sample size of our study was considered a significant limitation. The safety profile of the Sputnik V vaccine compared to other vaccines and among other populations should be evaluated in further studies with larger sample sizes across the country’s different regions.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted in accordance with the principles stated in the Declaration of Helsinki. All participants who completed the questionnaires were requested to provide written informed consent as a requirement for their participation in this study. The research protocol and written consent forms were reviewed and approved by the Ethics Committee of the Tehran University of Medical Sciences (Code: IR.TUMS.IKHC.REC.1400.175).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and supervision: Maliheh Hasannezhad, Hossein Khalili and Hamid Emadi Koochak; Methodology: Maliheh Hasannezhad and Mohammad Reza Salehi; Data analysis: Hossein Khalili and Esmaeil Mohammadnejad; Investigation and writing the original draft: Seyed Ali Dehghan Manshadi, Negar Toroghi, Anahid Nourian, Keyhan Mohammadi and Nasim Shirazi; Review and editing: Maliheh Hasannezhad and Elnaz Shahmohamadi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the Imam Khomeini Hospital Complex staff for their help.
References
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270-3. [DOI:10.1038/s41586-020-2012-7] [PMID]
- Kordzadeh-Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID-19). Future Microbiology. 2020; 15:1287-305. [DOI:10.2217/fmb-2020-0110] [PMID]
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents. 2020; 55(3):105924. [DOI:10.1016/j.ijantimicag.2020.105924] [PMID]
- Singhal T. A review of coronavirus disease-2019 (covid-19). Indian Journal of Pediatrics. 2020; 87(4):281-6. [DOI:10.1007/s12098-020-03263-6] [PMID]
- Sharif AS. Transmission routes of covid-19: A review of the evidence. Journal of Pediatric Nephrology. 2020; 8(2):1-4. [Link]
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020; 158(6):1831-3.e3. [DOI:10.1053/j.gastro.2020.02.055] [PMID]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020; 382(18):1708-20. [DOI:10.1056/NEJMoa2002032] [PMID]
- Wang C, Wang Z, Wang G, Lau JY, Zhang K, Li W. COVID-19 in early 2021: Current status and looking forward. Signal Transduction and Targeted Therapy. 2021; 6(1):114. [DOI:10.1038/s41392-021-00527-1] [PMID]
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (covid-19): A review. JAMA. 2020; 324(8):782-93. [DOI:10.1001/jama.2020.12839] [PMID]
- Jamshaid H, Zahid F, Din IU, Zeb A, Choi HG, Khan GM, et al. Diagnostic and Treatment Strategies for COVID-19. AAPS PharmSciTech. 2020; 21(6):222. [DOI:10.1208/s12249-020-01756-3] [PMID]
- Zahid MN, Moosa MS, Perna S, Buti EB. A review on COVID-19 vaccines: Stages of clinical trials, mode of actions and efficacy. Arab Journal of Basic and Applied Sciences. 2021; 28(1):225-33. [DOI:10.1080/25765299.2021.1903144]
- Khazaee-Pool M, Naghibi SA. Designing and evaluating validity and reliability of the questionnaire concerning the Factors Affecting Person’s Intention of COVID-19 Prevention (FAPI-COP). Iranian Journal Of Health Sciences. 2022; 10(3):1-12. [DOI:10.18502/jhs.v10i3.10517]
- Hajj Hussein I, Chams N, Chams S, El Sayegh S, Badran R, Raad M, et al. Vaccines through centuries: Major cornerstones of global health. Frontiers in Public Health. 2015; 3:269. [DOI:10.3389/fpubh.2015.00269] [PMID]
- Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. Medicina (B Aires). 2021; 81(3):408-14. [PMID]
- Jarynowski A, Semenov A, Kamiński M, Belik V. Mild adverse events of sputnik V Vaccine in Russia: Social media content analysis of telegram via deep learning. Journal of Medical Internet Research. 2021; 23(11):e30529. [PMID]
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nature Reviews Immunology. 2021; 21(10):626-36. [DOI:10.1038/s41577-021-00592-1] [PMID]
- Nagy A, Alhatlani B. An overview of current COVID-19 vaccine platforms. Computational and Structural Biotechnology Journal. 2021; 19:2508-17. [DOI:10.1016/j.csbj.2021.04.061] [PMID]
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397(10275):671-81. [DOI:10.1016/S0140-6736(21)00234-8] [PMID]
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. The Lancet. 2020; 396(10255):887-97. [DOI:10.1016/S0140-6736(20)31866-3] [PMID]
- Montalti M, Soldà G, Di Valerio Z, Salussolia A, Lenzi J, Forcellini M, et al. ROCCA observational study: Early results on safety of Sputnik V vaccine (Gam-COVID-Vac) in the Republic of San Marino using active surveillance. EClinicalMedicine. 2021; 38:101027. [DOI:10.1016/j.eclinm.2021.101027] [PMID]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013; 310(20):2191-4. [DOI:10.1001/jama.2013.281053] [PMID]
- WHO. Causality assessment of an adverse event following immunization (AEFI): User manual for the revised WHO classification, 2nd ed., 2019 update. Geneva: WHO; 2021. [Link]
- Azimi M, Dehzad WM, Atiq MA, Bahain B, Asady A. Adverse effects of the covid-19 vaccine reported by lecturers and staff of Kabul University of Medical Sciences, Kabul, Afghanistan. Infection and Drug Resistance. 2021; 14:4077-83. [PMID]
- Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of covid-19 vaccine side effects among healthcare workers in the Czech Republic. Journal of Clinical Medicine. 2021; 10(7):1428.[DOI:10.3390/jcm10071428] [PMID]
- Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, et al. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines (Basel). 2021; 9(2):119. [DOI:10.3390/vaccines9020119] [PMID]
- Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, et al. Side effects and perceptions following covid-19 vaccination in Jordan: A randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021; 9(6):556. [DOI:10.3390/vaccines9060556] [PMID]
Type of Study: Original Article |
Subject:
Infectious Diseases and Tropical Medicine
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






