Volume 13, Issue 1 (Winter 2025)
Iran J Health Sci 2025, 13(1): 57-64 |
Back to browse issues page
Ethics code: IR.SEMUMS.REC.1400.28
Clinical trials code: not applied.
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Izadi S, Bitaraf M, Rahaei F, Naghibi Rokni S P, Mansori K, Memarian M. The Association Between Vitamin D Serum Level and Severity of COVID-19: A Cross-sectional Study. Iran J Health Sci 2025; 13 (1) :57-64
URL: http://jhs.mazums.ac.ir/article-1-962-en.html
URL: http://jhs.mazums.ac.ir/article-1-962-en.html
Shahrzad Izadi 

 , Masoume Bitaraf
, Masoume Bitaraf 

 , Fatemeh Rahaei
, Fatemeh Rahaei 

 , Seyedeh Pardis Naghibi Rokni
, Seyedeh Pardis Naghibi Rokni 

 , Kamyar Mansori
, Kamyar Mansori 

 , Mohammad Memarian *
, Mohammad Memarian * 




 , Masoume Bitaraf
, Masoume Bitaraf 

 , Fatemeh Rahaei
, Fatemeh Rahaei 

 , Seyedeh Pardis Naghibi Rokni
, Seyedeh Pardis Naghibi Rokni 

 , Kamyar Mansori
, Kamyar Mansori 

 , Mohammad Memarian *
, Mohammad Memarian * 


Department of Internal Medicine, Faculty of Medicine, Semnan University of Medical Sciences, Semnan, Iran. , dr.memarian20@gmail.com
Full-Text [PDF 724 kb]
(605 Downloads)
| Abstract (HTML) (1867 Views)
Full-Text: (764 Views)
Introduction
Coronavirus disease 2019 (COVID-19) is a pneumonic pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This disease was first detected in December 2019 in Wuhan (China) [1, 2]. Approximately 15% of cases progress to severe (pneumonic) disease [3]. COVID-19 affects different systems and organs of the body, including the pulmonary, cardiovascular, nervous, and renal systems. Therefore, it is necessary to find ways to reduce the risk of severe infection and its morbidity and mortality [4].
The immune system is influenced by several factors that may contribute to the risk of mortality related to COVID-19. Meanwhile, vitamins may play an important role in the proper response of the body’s immune system against infection and diseases [5, 6]. Vitamin D plays an essential role in modulating innate and acquired immune responses. Vitamin D can be synthesized in the human body with the help of sunlight or obtained from food sources. Serum 25-hydroxyvitamin D (25 [OH] D) level is a reliable index to evaluate serum vitamin D in the body [7, 8].
Studies have shown that adequate vitamin D levels may reduce the duration of hospitalization and the severity of respiratory diseases [9, 10]. Recent studies have highlighted the important role of vitamin D in supporting immune system function, especially in balancing the inflammatory response to viral infection [11, 12]. For example, a cohort study demonstrates that serum vitamin D levels are lower in SARS-CoV-2 positive cases than negative ones [13]. Similarly, in another study, the reduction of serum vitamin D levels shows a significant relationship with the severity of complications in COVID-19 patients [14]. From both epidemiological data and biochemical and immunological evidence, vitamin D may be an important modulating factor in the severity of COVID-19 and other respiratory infectious diseases [15, 16]. However, based on our knowledge, studies on the role of vitamin D supplementation in reducing the risk of contracting COVID-19 are limited. Therefore, considering the limitations of the studies and the controversial effect of vitamin D serum level on the risk of infection, we conducted this study to determine the association between vitamin D serum level and severity of COVID-19 in Iran.
Materials and Methods
Study design and subjects
This cross-sectional study was performed to determine the association between vitamin D serum level and the severity of COVID-19 in patients referred to Kowsar Hospital of Semnan City, Iran, in 2022. The samples were recruited by convenience sampling. A total of 121 COVID-19 patients based on lung CT or polymerase chain reaction (PCR) tests were assigned to mild/moderate (n=80) and severe (n=41) COVID-19 groups. The inclusion criteria comprised age ≥18 years, diagnosis of COVID-19 based on lung computed tomography (CT) scan or positive PCR test of nasopharyngeal swab sample, and willingness to participate in the research. The exclusion criteria consisted of lack of consent to participate in the study, parathyroid disorders, kidney failure, chronic liver disease, and liver cirrhosis.
Data collection
In the current study, the diagnosis of COVID-19 was based on lung CT or PCR test. Then, based on Centers for Disease Control and Prevention (CDC) criteria, the patients with a fever 5-6 days after infection and mild respiratory symptoms were categorized as mild/moderate COVID-19, and patients with symptoms of shortness of breath, respiratory rate more than 30 per minute, hypoxia, SpO2 <93% and or lung penetration >50% of the lung field during 24 to 48 hours were classified as severe COVID-19. Also, patients with symptoms of respiratory failure, multiple organ dysfunction/failure, and septic shock were considered as severe COVID-19.
The data collection tool was a checklist including the variables of age, sex, body mass index (BMI), erythrocyte sedimentation rate (ESR), length of hospitalization, SpO2, C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), vitamin D level, smoking history, underlying disease, outcome, duration of hospitalization in intensive care unit (ICU), intubation and hospitalization complications were extracted through interview and the patient’s medical file. However, to measure the serum level of vitamin D, 10 mL of blood was taken from all patients with COVID-19, and then the level of this vitamin was measured by liquid chromatography-tandem mass spectrometry method.
Statistical analysis
Data were analyzed using Stata software, version 14. The Mean±SD, and percentages were reported for descriptive analyses. Then, the univariate and multivariate logistic regression model was used to determine the relationship between vitamin D serum level and other variables under study with the severity of COVID-19.
Results
A total of 121 COVID-19 patients were examined in the mild/moderate (n=80) and severe (n=41) groups. Tables 1 and 2 present patients’ demographic and clinical characteristics in mild/moderate and severe groups.
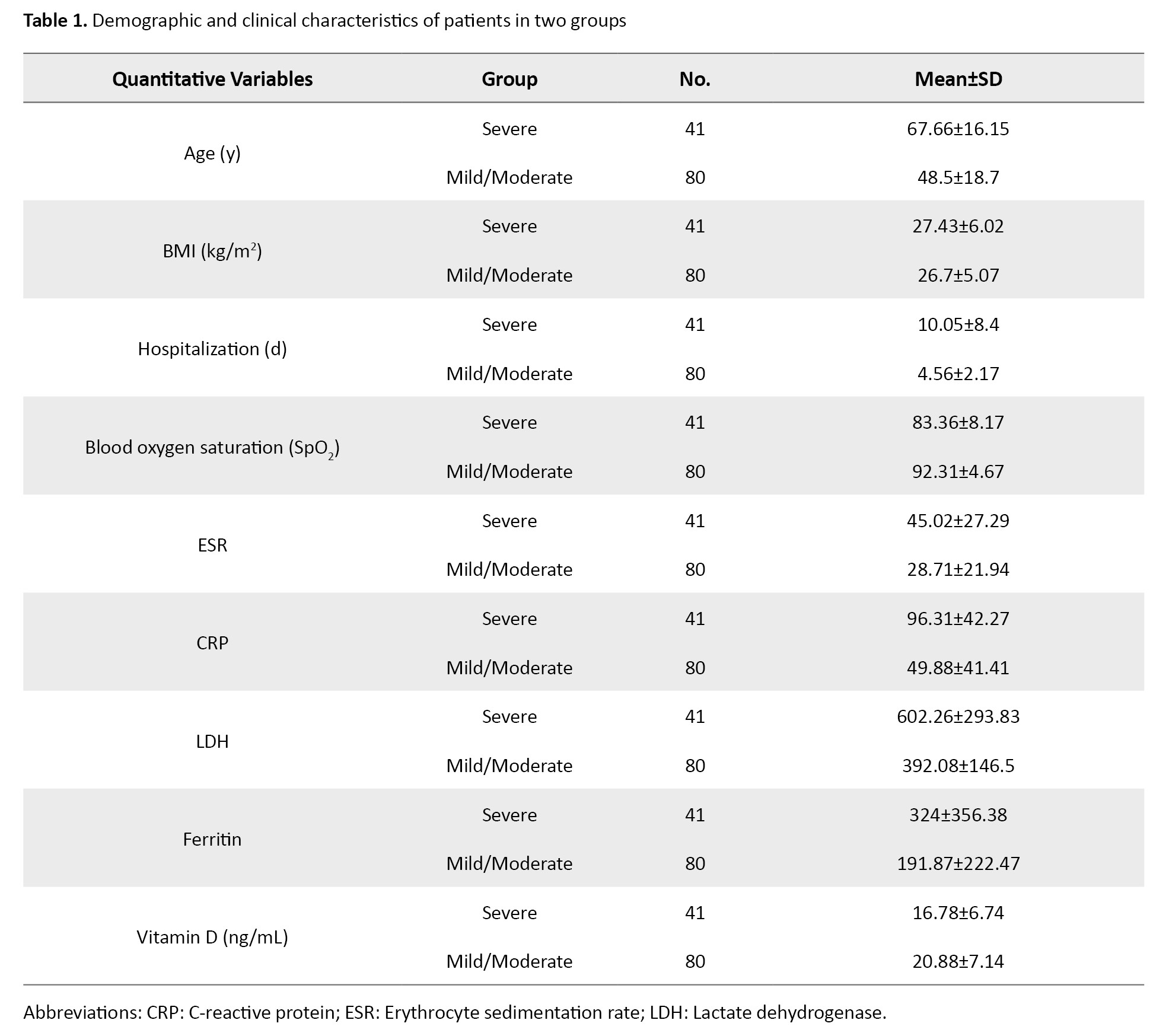

The mean age of severe and mild/moderate groups were 67.66±16.15 and 48.5±18.7 years, respectively. The numbers (%) of males were 25(61%) and 44(55%) severe and mild/moderate groups, respectively. The Mean±SD values of SpO2, ESR, CRP, LDH, and ferritin in the mild/moderate vs severe COVID-19 groups were 83.36±8.17 vs 92.31±4.67, 45.02±27.29 vs 28.71±21.94, 96.31±42.27 vs 49.88±41.41, 602.26±293.83 vs 392.08±146.5 and 324±356.38 vs 191.87±222.47, respectively. Also, the mean vitamin D levels of severe and mild/moderate groups were 16.78±6.74 and 20.88±7.14 ng/mL, respectively. The percentages of smokers in the two groups was 17(41.5%) and 20(25%), respectively.
Table 3 presents the predictive factors of the severity of COVID-19 by univariate logistic regression model.
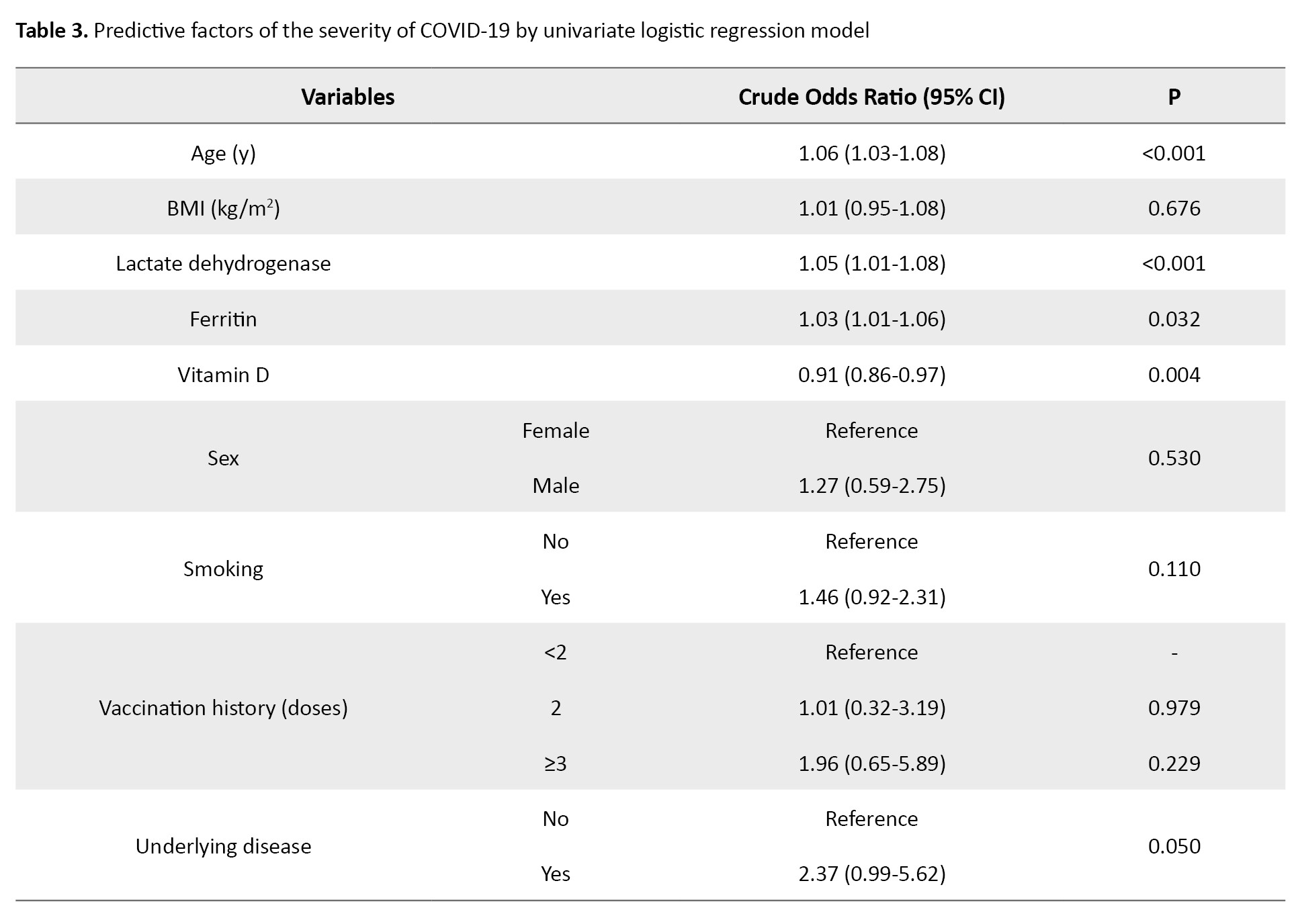
Age, vitamin D, LDH, ferritin, smoking, and underlying disease were the important factors predicting the severity of COVID-19 (P≤0.20).
Table 4 shows the odds ratio and 95% CI derived from a multivariate logistic regression model for the predictive factors of the severity of COVID-19.

After adjusting for the confounding variables, vitamin D (odds ratio [OR]=0.55; 95% CI, 0.42%, 0.83%) was the most important factor predicting the severity of COVID-19 so that, for one ng/mL increase in vitamin D level, the odds of contracting the severe form of COVID-19 decreases by about 45%.
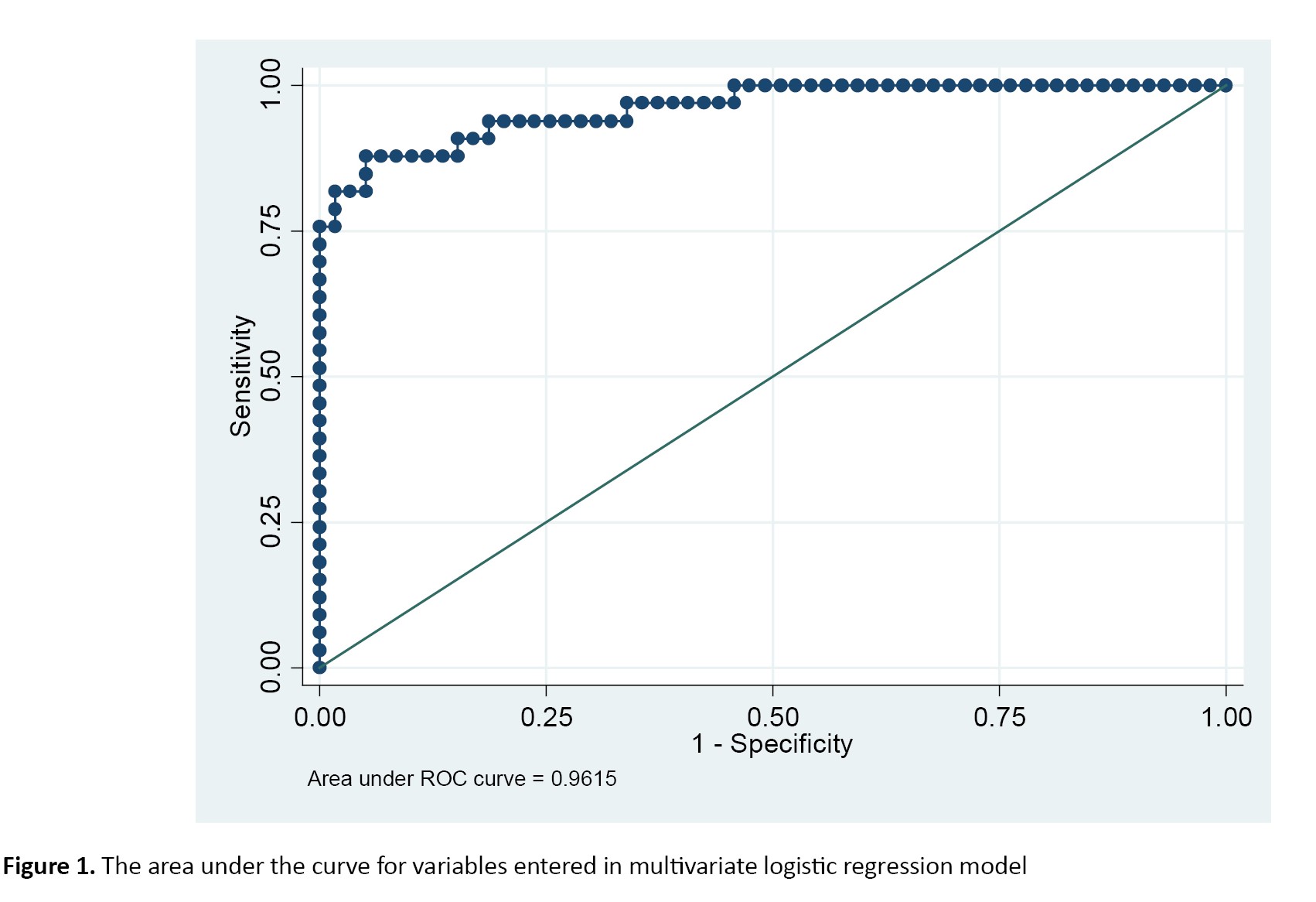
The area under the ROC curve was 0.9515 for significant variables included in the multivariate logistic regression model, demonstrating this model’s high discriminative power (Figure 1).
Discussion
This present study was conducted to determine the association between vitamin D serum levels and the severity of COVID-19 in Iran. Our results show that the mean age of severe and mild/moderate groups were 67.66±16.15 and 48.5±18.7 years, respectively.
The percentages of males were 25(61%) and 44(55%) in the severe and mild/moderate groups, respectively. The multivariate logistic regression model shows that vitamin D (OR=0.55; 95% CI, 0.42%, 0.83%) was the most important factor predicting the severity of COVID-19 so that, for one ng/mL increase in vitamin D level, the odds of contracting the severe form of COVID-19 decreases by about 45%.
Vitamin D is a steroid hormone produced endogenously by the action of ultraviolet rays on the skin. It is also available from exogenous food sources or dietary supplements [17]. Recently, various studies have shown a potential association between vitamin D deficiency and respiratory infections [17, 18]. Vitamin D deficiency affects the functions of the immune system and increases innate immunity by secreting antiviral peptides and improving mucosal defenses [19, 20]. Studies have demonstrated that low serum vitamin D levels may contribute to respiratory infections [21, 22]. In a meta-analysis study, vitamin D serum levels less than <20 ng/mL increased the risk of pneumonia by 64% [23].
In line with our study, some studies have suggested that vitamin D deficiency may compromise the function of the respiratory immune system and the risk of severe COVID-19 and related mortality [24]. Also, some observational studies have shown the correlation between vitamin D with severity and mortality in COVID-19 patients [25-27]. In a meta-analysis study of 1403715 COVID-19 patients, low vitamin D levels are associated with increased ICU hospitalization, disease severity, and mortality from COVID-19 [28]. D’Avolio et al. and Radujkovic et al. suggested that the vitamin D level was lower in people with a positive PCR test than in a negative PCR [13, 29]. Other studies have also shown an inverse relationship between vitamin D serum levels and levels of interleukin-6 and CRP in pneumonia and acute respiratory distress syndrome (ARDS) [23, 30, 31].
Vitamin D has an important role in immune system function, especially in balancing the inflammatory response to viral infection. It has been suggested that vitamin D can reduce the risk of infection through several mechanisms [11, 12]. These mechanisms include the induction of cathelicidins and defensins, which can reduce the speed of virus replication and the concentration of pro-inflammatory cytokines [32]. Vitamin D mechanisms in reducing the risk of viral infections and mortality are different, as mentioned. For example, to reduce the risk of colds, vitamin D uses three pathways of physical barrier, natural cellular immunity, and adaptive immunity to increase immunity. The study of Grant et al. showed that vitamin D supplementation can reduce the risk of influenza and COVID-19 infection and mortality [32]. This vitamin plays a role in reducing viral infections by increasing cellular immunity and reducing cytokine storm by affecting interferon γ and tumor necrosis factor α and regulating adaptive immunity by inhibiting T helper cell type 1 responses and stimulating the induction of T cells [33]. Talmor’s study also shows that calcitriol 1 25 OH 2D inhibits the production of pro-inflammatory cytokines such as interleukin-2, interferon-gamma, and tumor necrosis factor-alpha [34]. Evidence suggests that calcitriol 1,25 OH 2D leads to upregulating nuclear factor kappa B inhibitors in respiratory syncytial virus-infected A549 alveolar cells and primary human tracheobronchial epithelial cells. In addition, vitamin D increases the expression of angiotensin-converting enzyme 2, the main host cell receptor of COVID-19, and also downregulates renin at the transcriptional level [35]. The combination of vitamin D effects on the inflammatory pathway and angiotensin-converting enzyme 2 expression may be uniquely relevant to the pathogenesis and severity of COVID-19 [36].
Conclusion
Our study showed that the serum level of vitamin D is an important predictor of the severity of COVID-19, so a decrease in this vitamin can increase the risk of severe COVID-19. However, in order to confirm this finding, it is recommended that detailed studies with a larger sample size be conducted in this field.
Study limitations
The most important limitation was the low sample size and the unequal number of patients in the two groups of mild/moderate and severe COVID-19, which can reduce the statistical efficiency. The second limitation is the study’s cross-sectional nature and the one-time vitamin D serum level measurement, which necessitates conducting cohort studies with a large sample size.
Ethical Considerations
Compliance with ethical guidelines
This study was performed according to the principles expressed in the Declaration of Helsinki and was approved by the Deputy of the Research and Ethics Committee of Semnan University of Medical Sciences, Semnan, Iran (Code: IR.SEMUMS.REC.1400.283).
Funding
This study was extracted from the General Medical Doctorate thesis of Seyedeh Pardis Naghibi Rokni, approved by the Faculty of Medicine, Semnan University of Medical Sciences, Semnan, Iran.
Authors contributions
Data collection, statistical analysis: Shahrzad Izadi and Mohammad Memarian; Study design, and data analysis: Masoume Bitaraf, Fatemeh Rahaei, and Seyedeh Pardis Naghibi Rokni; Conceptualization, review and editing: Kamyar Mansori, Shahrzad Izadi and Mohammad Memarian; Writing the original draft and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to thank the staff and rheumatic patients of Kowsar Hospital of Semnan for their sincere cooperation in conducting this research.
References
Coronavirus disease 2019 (COVID-19) is a pneumonic pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This disease was first detected in December 2019 in Wuhan (China) [1, 2]. Approximately 15% of cases progress to severe (pneumonic) disease [3]. COVID-19 affects different systems and organs of the body, including the pulmonary, cardiovascular, nervous, and renal systems. Therefore, it is necessary to find ways to reduce the risk of severe infection and its morbidity and mortality [4].
The immune system is influenced by several factors that may contribute to the risk of mortality related to COVID-19. Meanwhile, vitamins may play an important role in the proper response of the body’s immune system against infection and diseases [5, 6]. Vitamin D plays an essential role in modulating innate and acquired immune responses. Vitamin D can be synthesized in the human body with the help of sunlight or obtained from food sources. Serum 25-hydroxyvitamin D (25 [OH] D) level is a reliable index to evaluate serum vitamin D in the body [7, 8].
Studies have shown that adequate vitamin D levels may reduce the duration of hospitalization and the severity of respiratory diseases [9, 10]. Recent studies have highlighted the important role of vitamin D in supporting immune system function, especially in balancing the inflammatory response to viral infection [11, 12]. For example, a cohort study demonstrates that serum vitamin D levels are lower in SARS-CoV-2 positive cases than negative ones [13]. Similarly, in another study, the reduction of serum vitamin D levels shows a significant relationship with the severity of complications in COVID-19 patients [14]. From both epidemiological data and biochemical and immunological evidence, vitamin D may be an important modulating factor in the severity of COVID-19 and other respiratory infectious diseases [15, 16]. However, based on our knowledge, studies on the role of vitamin D supplementation in reducing the risk of contracting COVID-19 are limited. Therefore, considering the limitations of the studies and the controversial effect of vitamin D serum level on the risk of infection, we conducted this study to determine the association between vitamin D serum level and severity of COVID-19 in Iran.
Materials and Methods
Study design and subjects
This cross-sectional study was performed to determine the association between vitamin D serum level and the severity of COVID-19 in patients referred to Kowsar Hospital of Semnan City, Iran, in 2022. The samples were recruited by convenience sampling. A total of 121 COVID-19 patients based on lung CT or polymerase chain reaction (PCR) tests were assigned to mild/moderate (n=80) and severe (n=41) COVID-19 groups. The inclusion criteria comprised age ≥18 years, diagnosis of COVID-19 based on lung computed tomography (CT) scan or positive PCR test of nasopharyngeal swab sample, and willingness to participate in the research. The exclusion criteria consisted of lack of consent to participate in the study, parathyroid disorders, kidney failure, chronic liver disease, and liver cirrhosis.
Data collection
In the current study, the diagnosis of COVID-19 was based on lung CT or PCR test. Then, based on Centers for Disease Control and Prevention (CDC) criteria, the patients with a fever 5-6 days after infection and mild respiratory symptoms were categorized as mild/moderate COVID-19, and patients with symptoms of shortness of breath, respiratory rate more than 30 per minute, hypoxia, SpO2 <93% and or lung penetration >50% of the lung field during 24 to 48 hours were classified as severe COVID-19. Also, patients with symptoms of respiratory failure, multiple organ dysfunction/failure, and septic shock were considered as severe COVID-19.
The data collection tool was a checklist including the variables of age, sex, body mass index (BMI), erythrocyte sedimentation rate (ESR), length of hospitalization, SpO2, C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), vitamin D level, smoking history, underlying disease, outcome, duration of hospitalization in intensive care unit (ICU), intubation and hospitalization complications were extracted through interview and the patient’s medical file. However, to measure the serum level of vitamin D, 10 mL of blood was taken from all patients with COVID-19, and then the level of this vitamin was measured by liquid chromatography-tandem mass spectrometry method.
Statistical analysis
Data were analyzed using Stata software, version 14. The Mean±SD, and percentages were reported for descriptive analyses. Then, the univariate and multivariate logistic regression model was used to determine the relationship between vitamin D serum level and other variables under study with the severity of COVID-19.
Results
A total of 121 COVID-19 patients were examined in the mild/moderate (n=80) and severe (n=41) groups. Tables 1 and 2 present patients’ demographic and clinical characteristics in mild/moderate and severe groups.


The mean age of severe and mild/moderate groups were 67.66±16.15 and 48.5±18.7 years, respectively. The numbers (%) of males were 25(61%) and 44(55%) severe and mild/moderate groups, respectively. The Mean±SD values of SpO2, ESR, CRP, LDH, and ferritin in the mild/moderate vs severe COVID-19 groups were 83.36±8.17 vs 92.31±4.67, 45.02±27.29 vs 28.71±21.94, 96.31±42.27 vs 49.88±41.41, 602.26±293.83 vs 392.08±146.5 and 324±356.38 vs 191.87±222.47, respectively. Also, the mean vitamin D levels of severe and mild/moderate groups were 16.78±6.74 and 20.88±7.14 ng/mL, respectively. The percentages of smokers in the two groups was 17(41.5%) and 20(25%), respectively.
Table 3 presents the predictive factors of the severity of COVID-19 by univariate logistic regression model.

Age, vitamin D, LDH, ferritin, smoking, and underlying disease were the important factors predicting the severity of COVID-19 (P≤0.20).
Table 4 shows the odds ratio and 95% CI derived from a multivariate logistic regression model for the predictive factors of the severity of COVID-19.

After adjusting for the confounding variables, vitamin D (odds ratio [OR]=0.55; 95% CI, 0.42%, 0.83%) was the most important factor predicting the severity of COVID-19 so that, for one ng/mL increase in vitamin D level, the odds of contracting the severe form of COVID-19 decreases by about 45%.
The area under the ROC curve was 0.9515 for significant variables included in the multivariate logistic regression model, demonstrating this model’s high discriminative power (Figure 1).
Discussion
This present study was conducted to determine the association between vitamin D serum levels and the severity of COVID-19 in Iran. Our results show that the mean age of severe and mild/moderate groups were 67.66±16.15 and 48.5±18.7 years, respectively.
The percentages of males were 25(61%) and 44(55%) in the severe and mild/moderate groups, respectively. The multivariate logistic regression model shows that vitamin D (OR=0.55; 95% CI, 0.42%, 0.83%) was the most important factor predicting the severity of COVID-19 so that, for one ng/mL increase in vitamin D level, the odds of contracting the severe form of COVID-19 decreases by about 45%.
Vitamin D is a steroid hormone produced endogenously by the action of ultraviolet rays on the skin. It is also available from exogenous food sources or dietary supplements [17]. Recently, various studies have shown a potential association between vitamin D deficiency and respiratory infections [17, 18]. Vitamin D deficiency affects the functions of the immune system and increases innate immunity by secreting antiviral peptides and improving mucosal defenses [19, 20]. Studies have demonstrated that low serum vitamin D levels may contribute to respiratory infections [21, 22]. In a meta-analysis study, vitamin D serum levels less than <20 ng/mL increased the risk of pneumonia by 64% [23].
In line with our study, some studies have suggested that vitamin D deficiency may compromise the function of the respiratory immune system and the risk of severe COVID-19 and related mortality [24]. Also, some observational studies have shown the correlation between vitamin D with severity and mortality in COVID-19 patients [25-27]. In a meta-analysis study of 1403715 COVID-19 patients, low vitamin D levels are associated with increased ICU hospitalization, disease severity, and mortality from COVID-19 [28]. D’Avolio et al. and Radujkovic et al. suggested that the vitamin D level was lower in people with a positive PCR test than in a negative PCR [13, 29]. Other studies have also shown an inverse relationship between vitamin D serum levels and levels of interleukin-6 and CRP in pneumonia and acute respiratory distress syndrome (ARDS) [23, 30, 31].
Vitamin D has an important role in immune system function, especially in balancing the inflammatory response to viral infection. It has been suggested that vitamin D can reduce the risk of infection through several mechanisms [11, 12]. These mechanisms include the induction of cathelicidins and defensins, which can reduce the speed of virus replication and the concentration of pro-inflammatory cytokines [32]. Vitamin D mechanisms in reducing the risk of viral infections and mortality are different, as mentioned. For example, to reduce the risk of colds, vitamin D uses three pathways of physical barrier, natural cellular immunity, and adaptive immunity to increase immunity. The study of Grant et al. showed that vitamin D supplementation can reduce the risk of influenza and COVID-19 infection and mortality [32]. This vitamin plays a role in reducing viral infections by increasing cellular immunity and reducing cytokine storm by affecting interferon γ and tumor necrosis factor α and regulating adaptive immunity by inhibiting T helper cell type 1 responses and stimulating the induction of T cells [33]. Talmor’s study also shows that calcitriol 1 25 OH 2D inhibits the production of pro-inflammatory cytokines such as interleukin-2, interferon-gamma, and tumor necrosis factor-alpha [34]. Evidence suggests that calcitriol 1,25 OH 2D leads to upregulating nuclear factor kappa B inhibitors in respiratory syncytial virus-infected A549 alveolar cells and primary human tracheobronchial epithelial cells. In addition, vitamin D increases the expression of angiotensin-converting enzyme 2, the main host cell receptor of COVID-19, and also downregulates renin at the transcriptional level [35]. The combination of vitamin D effects on the inflammatory pathway and angiotensin-converting enzyme 2 expression may be uniquely relevant to the pathogenesis and severity of COVID-19 [36].
Conclusion
Our study showed that the serum level of vitamin D is an important predictor of the severity of COVID-19, so a decrease in this vitamin can increase the risk of severe COVID-19. However, in order to confirm this finding, it is recommended that detailed studies with a larger sample size be conducted in this field.
Study limitations
The most important limitation was the low sample size and the unequal number of patients in the two groups of mild/moderate and severe COVID-19, which can reduce the statistical efficiency. The second limitation is the study’s cross-sectional nature and the one-time vitamin D serum level measurement, which necessitates conducting cohort studies with a large sample size.
Ethical Considerations
Compliance with ethical guidelines
This study was performed according to the principles expressed in the Declaration of Helsinki and was approved by the Deputy of the Research and Ethics Committee of Semnan University of Medical Sciences, Semnan, Iran (Code: IR.SEMUMS.REC.1400.283).
Funding
This study was extracted from the General Medical Doctorate thesis of Seyedeh Pardis Naghibi Rokni, approved by the Faculty of Medicine, Semnan University of Medical Sciences, Semnan, Iran.
Authors contributions
Data collection, statistical analysis: Shahrzad Izadi and Mohammad Memarian; Study design, and data analysis: Masoume Bitaraf, Fatemeh Rahaei, and Seyedeh Pardis Naghibi Rokni; Conceptualization, review and editing: Kamyar Mansori, Shahrzad Izadi and Mohammad Memarian; Writing the original draft and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to thank the staff and rheumatic patients of Kowsar Hospital of Semnan for their sincere cooperation in conducting this research.
References
- Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clinical and Experimental Pediatrics. 2020; 63(4):119-24. [DOI:10.3345/cep.2020.00493] [PMID]
- Sohrabi C, Alsafi Z, O’neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). International Journal of Surgery. 2020; 76:71-6. [DOI:10.1016/j.ijsu.2020.02.034] [PMID]
- Verma S, Carter EB, Mysorekar IU. SARS-CoV2 and pregnancy: An invisible enemy? American Journal of Reproductive Immunology. 2020; 84(5):e13308. [DOI:10.1111/aji.13308] [PMID]
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323(20):2052-9. [DOI:10.1001/jama.2020.6775] [PMID]
- Banerjee A, Ganguly U, Saha S, Chakrabarti S, Saini RV, Rawal RK, et al. Vitamin D and immuno-pathology of COVID-19: Many interactions but uncertain therapeutic benefits. Expert Review of Anti-Infective Therapy. 2021; 19(10):1245-58. [DOI:10.1080/14787210.2021.1905519] [PMID]
- Patel N, Penkert RR, Jones BG, Sealy RE, Surman SL, Sun Y, et al. Baseline serum vitamin A and D levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019; 11(10):907. [DOI:10.3390/v11100907] [PMID]
- Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Annals of Nutrition and Metabolism. 2007; 51(4):301-23. [DOI:10.1159/000107673] [PMID]
- Christopher KB. Vitamin D and critical illness outcomes. Current Opinion in Critical Care. 2016; 22(4):332-8. [DOI:10.1097/MCC.0000000000000328] [PMID]
- Maghbooli Z, Sahraian MA, Ebrahimi M, Pazoki M, Kafan S, Tabriz HM, et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. Plos One. 2020; 15(9):e0239799. [DOI:10.1371/journal.pone.0239799] [PMID]
- Panagiotou G, Tee SA, Ihsan Y, Athar W, Marchitelli G, Kelly D, et al. Low serum 25-hydroxyvitamin D (25 [OH] D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clinical Endocrinology. 2020; 93(4):508-11. [PMID]
- Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. Journal of Clinical Virology. 2011; 50(3):194-200. [DOI:10.1016/j.jcv.2010.12.006] [PMID]
- Mathyssen C, Aelbrecht C, Serré J, Everaerts S, Maes K, Gayan-Ramirez G, et al. Local expression profiles of vitamin D-related genes in airways of COPD patients. Respiratory Research. 2020; 21(1):137. [DOI:10.1186/s12931-020-01405-0] [PMID]
- D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020; 12(5):1359.[DOI:10.3390/nu12051359] [PMID]
- Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van den Abbeele K, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgraduate Medical Journal. 2021; 997(1149):442-7. [DOI:10.1136/postgradmedj-2020-138712] [PMID]
- Jevalikar G, Mithal A, Singh A, Sharma R, Farooqui KJ, Mahendru S, et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Scientific Reports. 2021; ;11(1):6258. [DOI:10.1038/s41598-021-85809-y] [PMID]
- Pham H, Rahman A, Majidi A, Waterhouse M, Neale RE. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health. 2019; 16(17):3020.[DOI:10.3390/ijerph16173020] [PMID]
- Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Reviews in Endocrine and Metabolic Disorders. 2017; 18(2):153-65. [DOI:10.1007/s11154-017-9424-1] [PMID]
- Infante M, Ricordi C, Sanchez J, Clare-Salzler MJ, Padilla N, Fuenmayor V, et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019; 11(9):2185.[DOI:10.3390/nu11092185] [PMID]
- Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015; 7(6):4240-70. [DOI:10.3390/nu7064240] [PMID]
- Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, et al. Direct and indirect induction by 1, 25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. Journal of Biological Chemistry. 2010; 285(4):2227-31. [DOI:10.1074/jbc.C109.071225] [PMID]
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiology & Infection. 2006; 134(6):1129-40. [DOI:10.1017/S0950268806007175] [PMID]
- Cannell JJ, Vieth R, Willett W, Zasloff M, Hathcock JN, White JH, et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Annals of Otology, Rhinology & Laryngology. 2008; 117(11):864-70. [DOI:10.1177/000348940811701112] [PMID]
- Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine. 2019; 98(38):e17252. [DOI:10.1097/MD.0000000000017252] [PMID]
- Zeidan NMS, Lateef HMAE, Selim DM, Razek SA, Abd-Elrehim GAB, Nashat M, et al. Vitamin D deficiency and vitamin D receptor FokI polymorphism as risk factors for COVID-19. Pediatric Research. 2023; 93(5):1383-90. [DOI:10.1038/s41390-022-02275-6] [PMID]
- Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. MedRxiv. 2020. [DOI:10.1101/2020.04.08.20058578]
- Darling AL, Ahmadi KR, Ward KA, Harvey NC, Alves AC, Dunn-Walters DK, et al. Vitamin D status, body mass index, ethnicity and COVID-19: Initial analysis of the first-reported UK Biobank COVID-19 positive cases (n 580) compared with negative controls (n 723). MedRxiv. 2020. [DOI:10.1101/2020.04.29.20084277]
- De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Vitamin D deficiency as risk factor for severe COVID-19: A convergence of two pandemics. MedRxiv. 2020. [DOI:10.1101/2020.05.01.20079376]
- Chiodini I, Gatti D, Soranna D, Merlotti D, Mingiano C, Fassio A, et al. Vitamin D status and SARS-CoV-2 infection and COVID-19 clinical outcomes. Frontiers in Public Health. 2021; 9:736665.[DOI:10.3389/fpubh.2021.736665] [PMID]
- Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020; 12(9):2757. [DOI:10.3390/nu12092757] [PMID]
- Dancer RC, Parekh D, Lax S, D'Souza V, Zheng S, Bassford CR, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015; 70(7):617-24. [DOI:10.1136/thoraxjnl-2014-206680] [PMID]
- Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. New England Journal of Medicine. 2019; 381(6):520-30. [DOI:10.1056/NEJMoa1900906] [PMID]
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020; 12(4):988. [PMID]
- Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1, 25 (OH) 2D regulation of T cells. Nutrients. 2015; 7(4):3011-21. [DOI:10.3390/nu7043011] [PMID]
- Talmor Y, Bernheim J, Klein O, Green J, Rashid G. Calcitriol blunts pro-atherosclerotic parameters through NFκB and p38 in vitro. European Journal of Clinical Investigation. 2008; 38(8):548-54. [DOI:10.1111/j.1365-2362.2008.01977.x] [PMID]
- Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharideinduced acute lung injury via regulation of the reninangiotensin system. Molecular Medicine Reports. 2017; 16(5):7432-8. [DOI:10.3892/mmr.2017.7546] [PMID]
- Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflammation Research. 2020; 69(9):825-39. [DOI:10.1007/s00011-020-01372-8] [PMID]
Type of Study: Original Article |
Subject:
Nutrition
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |