Volume 12, Issue 4 (Autumn 2024)
Iran J Health Sci 2024, 12(4): 273-280 |
Back to browse issues page
Ethics code: IR.TUMS.CHMC.REC.1400.260
Clinical trials code: NA
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghanbari A, Pazoki M, Allahverdi B, Rohani P, Rahmani P. Profile of Children With Non-alcoholic Fatty Liver Disease Referred to a Hospital in Iran. Iran J Health Sci 2024; 12 (4) :273-280
URL: http://jhs.mazums.ac.ir/article-1-994-en.html
URL: http://jhs.mazums.ac.ir/article-1-994-en.html
Children’s Medical Center, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. , parisarahmani59@gmail.com
Keywords: Non-alcoholic fatty liver disease (NAFLD), Pediatric fatty liver, Clinical indicators, Pediatric obesity
Full-Text [PDF 918 kb]
(708 Downloads)
| Abstract (HTML) (2050 Views)
Full-Text: (567 Views)
Introduction
Hepatic steatosis, as fatty liver disease, refers to the accumulation of excess fat, especially triglycerides, within liver cells or hepatocytes [1]. Metabolic dysfunction-associated fatty liver disease is a new term suggested in 2020 for non-alcoholic fatty liver disease (NAFLD) as a systemic metabolic dysfunction [2]. When hepatocytes with fat droplets become inflamed and damaged, healthy cells respond by replacing the tissue damaged by the accumulation of triglycerides with normal hepatic tissue to repair the damage [3]. If this damaging process persists, the liver’s capacity to produce an adequate amount of healthy tissue will decrease, and fibrous tissue will take its place [4].
The NAFLD is becoming more widespread in children and adolescents worldwide, making it the most prevalent chronic liver disease in this age group, affecting 3-20% of them [5, 6]. Individuals with NAFLD may have vague symptoms or be asymptomatic, complicating the diagnosis process [7]. The most reliable method for the diagnosis of NAFLD in adults is liver biopsy due to its ability to provide accurate information about the extent of liver damage and the presence of inflammation [8]. Because of its invasive nature and the possibility of side effects, it may be challenging to be performed on children. Ultrasound is an alternate diagnostic tool for this purpose in children, offering a non-invasive way to assess liver health and detect abnormalities [9].
The NAFLDs, especially non-alcoholic steatohepatitis and fibrosis, are associated not only with liver-related problems but also with an increased risk of cardiovascular diseases, type 2 diabetes, and mortality in adulthood [10, 11]. The presence of NAFLD in children is more challenging since there are currently no specific non-invasive indicators available for the detection of liver inflammation in clinical settings [12]. Due to its chronic nature, NAFLD can negatively affect children’s health beyond their physical aspects. Obese children with NAFLD experience a lower quality of life compared to obese children without the NAFLD [13]. Children with NAFLD have more severe physical and psychological impairments in comparison with their healthy counterparts [13, 14].
The occurrence of NAFLD is increasing due to changes in lifestyle, such as a rise in sedentary behaviors and the consumption of high-calorie foods, although it is also often not diagnosed or identified [15, 16]. Hence, it is essential to implement early detection strategies, specifically targeting children with a greater susceptibility to this disease [17]. Given the longer lifespan of children with NAFLD compared to those with fatal diseases, they are more likely to experience the challenges associated with this condition for a longer period [18]. Therefore, it is crucial to have efficient screening methods for children who are at a higher risk of experiencing NAFLD. Screening for NAFLD is recommended as it enables the identification of the condition prior to the development of irreversible and advanced liver damage [17]. Gaining knowledge about the characteristics and control of NAFLD in children might provide a way to intervene early and modify the course of the condition. There is a lack of studies on the basic findings of children with NAFLD. Early detection of this disease may halt its development and alleviate its enduring adverse effects on the child’s well-being. Therefore, this study aims to identify the clinical indicators of NAFLD for children to manage its progression effectively and prevent complications.
Materials and Methods
Study design and participants
This is a descriptive-analytical study with a cross-sectional design conducted on children diagnosed with NAFLD referred to Children’s Medical Center in Tehran, Iran. All children with NAFLD at the Pediatric Gastroenterology and Hepatology Clinic of this hospital during 2021-2022 were included in the study (n=66). The NAFLD was diagnosed using ultrasound reports from expert radiologists. Inclusion criteria were age at diagnosis ≤18 years, diagnosis of NAFLD based on ultrasound or liver biopsy, elevation of alanine aminotransferase (ALT) level more than 1.5 times the upper limit of normal in obese or overweight children. Exclusion criteria were insufficient imaging evidence of NAFLD, presence of viral hepatitis, autoimmune hepatitis, Wilson’s disease, metabolic diseases, or genetic disorders leading to hepatic steatosis (e.g. glycogen storage disease), and presence of fatty liver due to alcohol consumption or use of steatogenic drugs.
Data collection
Demographic and laboratory data of patients were collected from the SABARA system (an electronic health record system used in Iran) using a checklist designed by the authors. The extracted data included patients’ demographic and laboratory data (age, gender, weight, body mass index [BMI], family history of fatty liver disease, fasting blood glucose level, serum ALT and aspartate transaminase [AST] levels, serum triglyceride [TG] level, total cholesterol [TC] level, history of formula feeding during infancy, history of breastfeeding during infancy), physicians’ prescriptions, and related factors. Information regarding patients’ lifestyles, including frequency of physical activity (PA) per week, frequency of eating fast food per week, and frequency of eating sweet foods per week, was gathered using a standard nutritional screening questionnaire for adolescents developed by the Iranian Ministry of Health and Medical Education in 2016 [19]. Based on this questionnaire, the PA level was classified into three categories: Very little or no purposeful PA, <420 minutes per week, and ≥420 minutes per week. Also, the frequency of eating fast foods and sweets was classified into three categories: Almost daily, weekly (once or twice per week), and rarely/never (once or twice per month). Accordingly, the daily screen time was classified into three categories: >2 hours per day, approximately 2 hours per day, and <2 hours per day.
Statistical analysis
Qualitative data were reported using frequency, while quantitative data were reported using the Mean±SD (if they had a normal distribution) and median and interquartile range (IQR) (if they had no normal distribution). If the data distribution was normal, to examine the difference between categorical variables, the chi-square test and ANOVA were used, followed by Fisher’s exact test, if necessary. To examine the difference between quantitative variables with binary qualitative variables, the independent sample t-test was used. In cases of abnormal data distribution, the equivalent non-parametric tests were used. All statistical analyses were performed in SPSS software, version 27. The significance level was set at 0.05.
Results
Participants’ demographic characteristics, weight and BMI
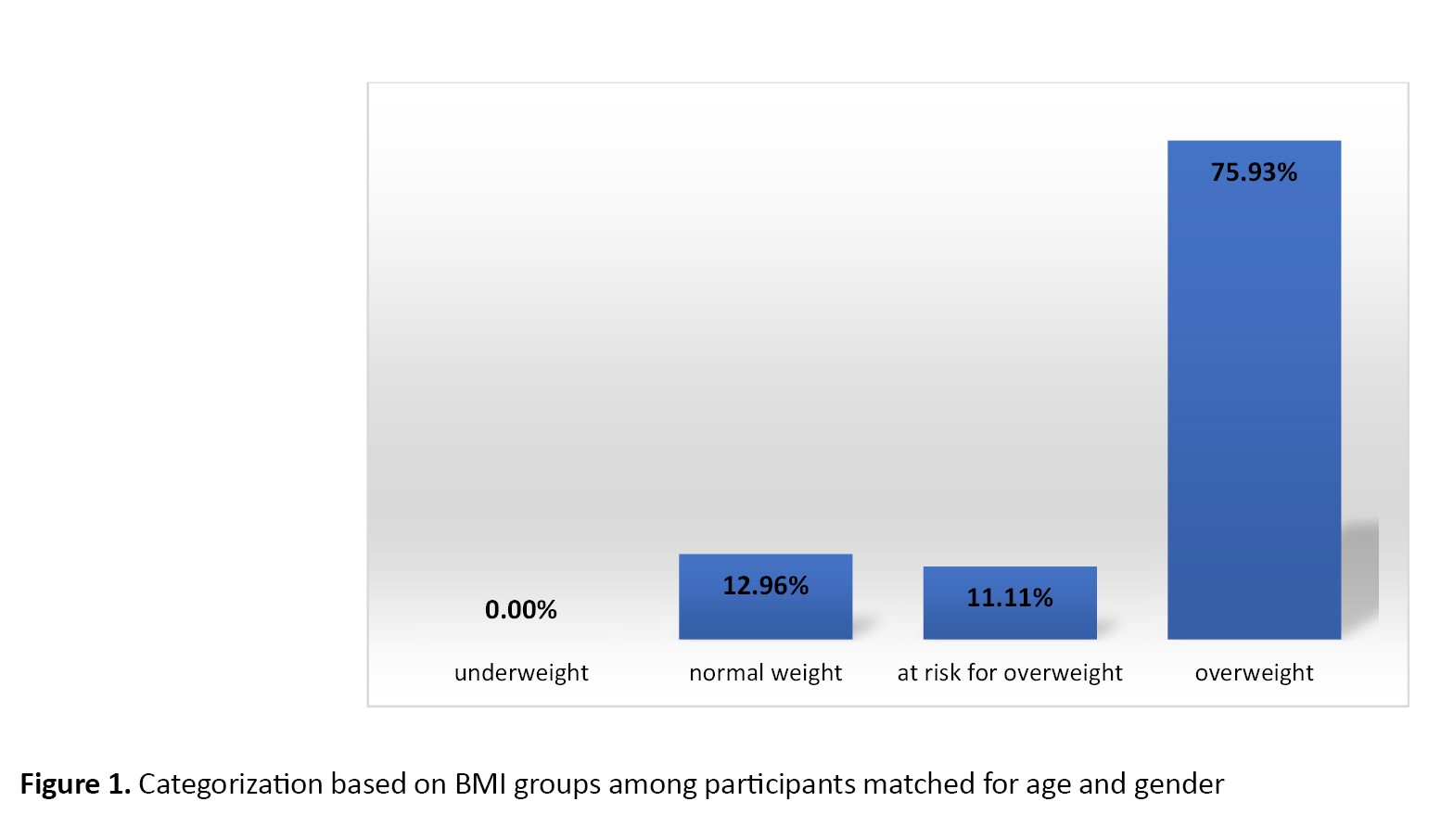
In this study, 66 children with NAFLD participated. The majority of them were boys (74.0%, n=49). Their mean age was 118.04±42.01 months, ranged 8-196 months. The mean weight and BMI were 57.37±28.4 kg and 26.37±7.41 kg/m2, respectively (Figure 1). Most of the children had a positive family history of fatty liver disease (57.4%, n=31 out of 54) and had a history of using infant formula before 6 months (24.1%, n=13 out of 54).
Participants’ PA, dietary habits, and screen time
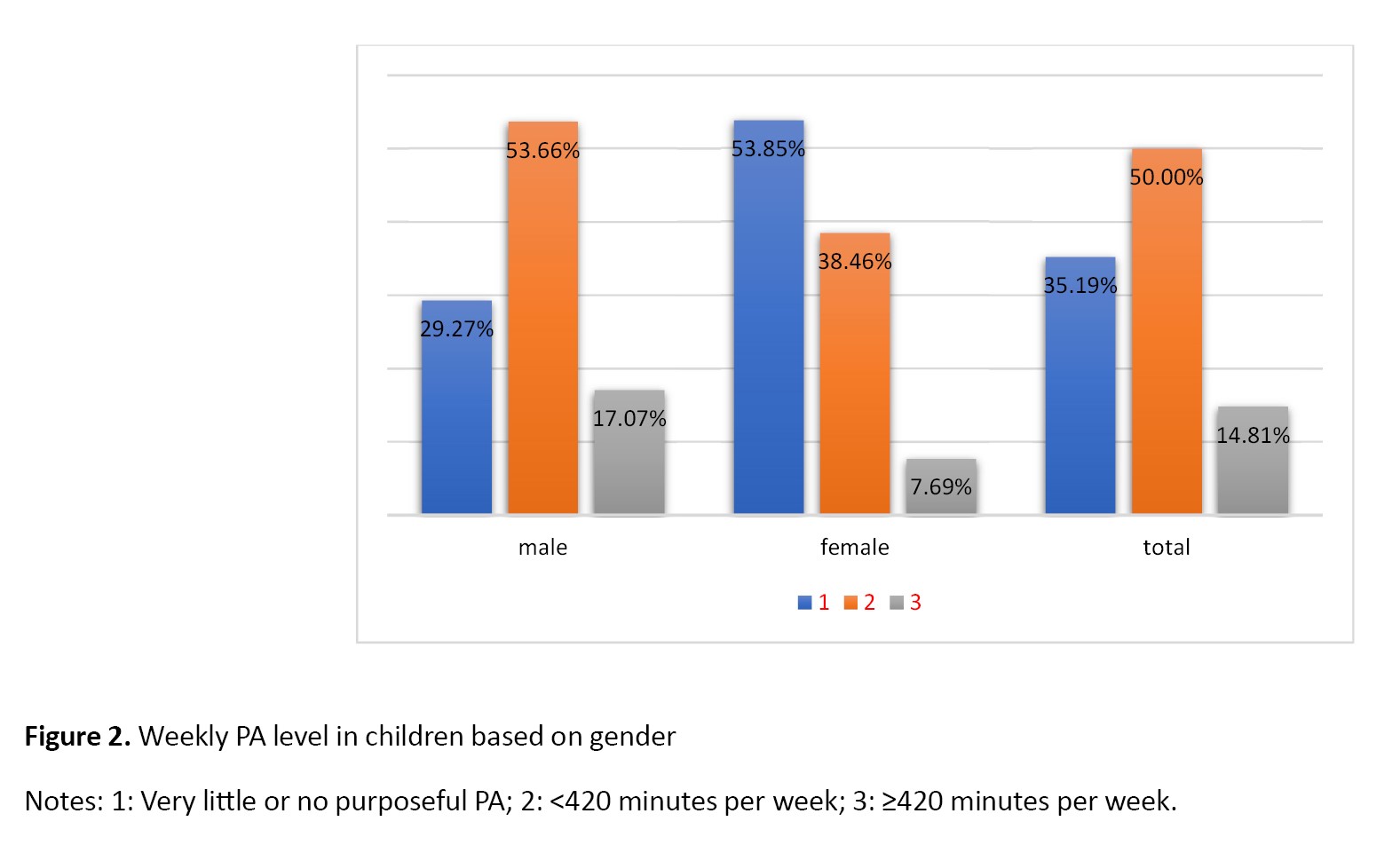
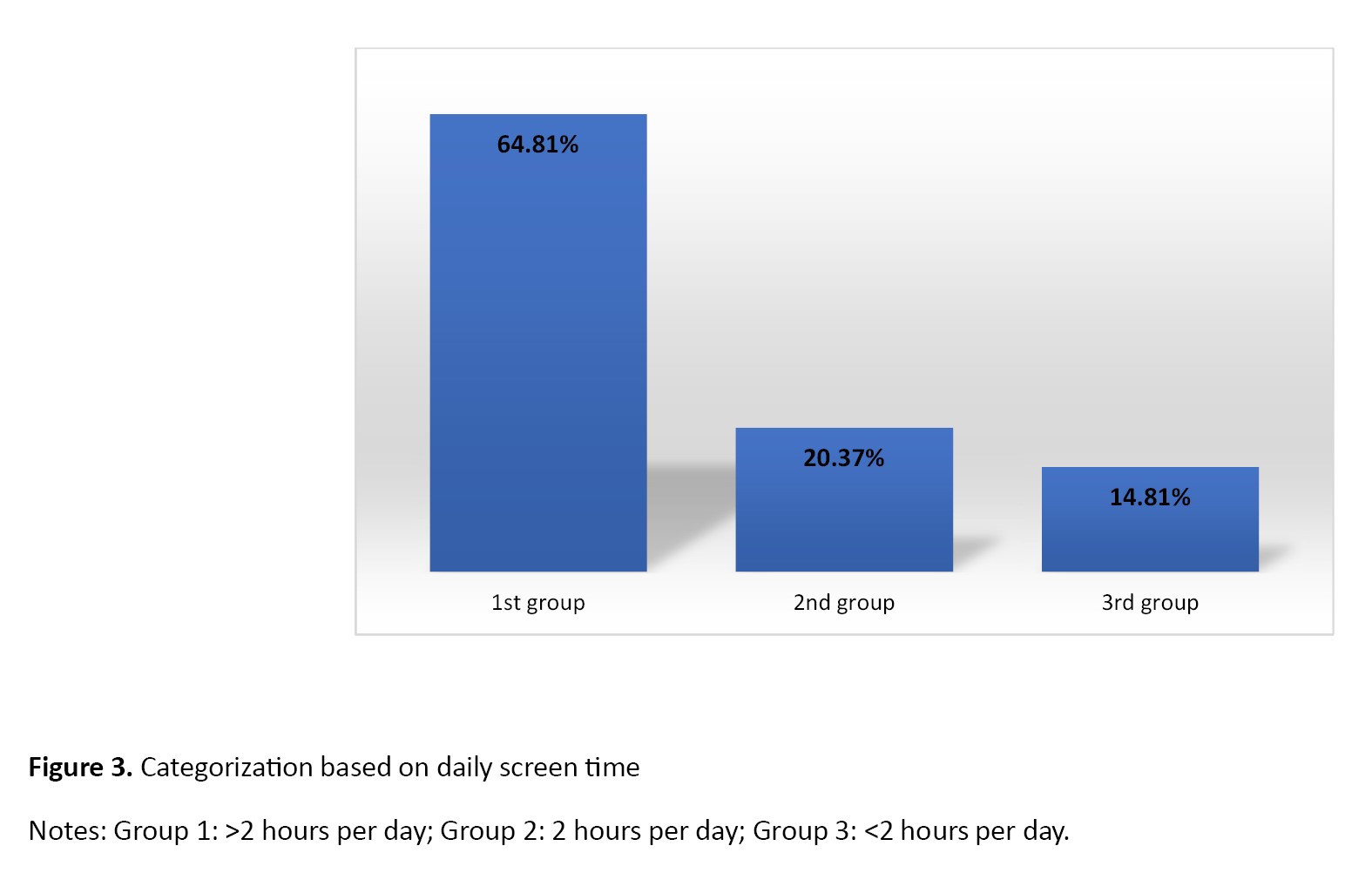
Based on the nutritional screening tool, 19 children (35.19%) were classified in the first group (very little or no purposeful PA), 27 children (50%) in the second group (<420 minutes per week), and 8 children (14.81%) in the third group (≥420 minutes per week). Additionally, based on gender, 29.27% (n=12) of boys and 53.85% of girls (n=7) were in the first group; 53.66% of boys (n=22) and 38.46% of girls (n=5) were in the second group, and 17.07% of boys (n=7) and 7.69% of girls (n=1) were in the third group (Figure 2). Regarding the frequency of eating sweet foods, according to the parents of children, 37 children (68.52%) were classified in the first group (almost daily), 7(12.96%) in the second group (weekly), and 10(18.52%) in the third group (rarely/never). Regarding the frequency of eating fast foods, 2 children (3.7%) were classified in the first group, 12(12.22%) in the second group, and 40(74.07%) in the third group. Overall, 37 children (68.52%) were classified in the first group, 7(12.96%) in the second group, and 10(18.52%) in the third group. Regarding the daily screen time, according to the parents of children, 35 children (64.81%) fell into the first group (>2 hours per day), 11 individuals (20.37%) into the second group (2 hours per day), and 8 individuals (14.81%) into the third group (<2 hours per day) (Figure 3).
Participants’ laboratory characteristics
AST and ALT Levels
The mean AST level for males was 37.28±15.71 U/L, and for females was 52.11±59.81 U/L. The median AST levels for males and females were 35 U/L and 34 U/L, respectively. The IQR for males and females was 22 and 26, respectively. Overall, the mean AST level in children was 41.55±34.74 U/L, with a median of 34 U/L and an IQR of 24. The difference in the AST level between males and females was not statistically significant (P=0.43).
Males had a mean ALT level of 57.69±40.46 U/L, whereas females had a higher mean level (62.52±96.34 U/L). The median ALT levels for males and females were 49 U/L and 32 U/L, respectively. The IQR of ALT was 41 in males and 48.50 in females. Overall, the mean ALT level was 59.06±60.72 U/L with a median of 45.5 U/L and an IQR of 44.5. The P for the difference in ALT levels between genders was 0.12, indicating no significant difference.
AST/ALT ratio
The AST/ALT ratio had a mean of 0.81±0.36 for males and 1.08±0.4 for females. The medians were 0.74 for males and 0.45 for females, with IQRs of 0.356 and 1.06, respectively. There was a significant difference in the AST/ALT ratio between males and females (P=0.01).
Participants with a family history of fatty liver disease had a mean AST/ALT ratio of 0.83±0.34, while those with no family history had a mean of 0.95±0.34. The medians were 0.78 for those with a family history and 0.43 for those with no family history, and their corresponding IQR were 0.28 and 0.92, respectively. The difference in the AST/ALT ratio based on the family history of fatty liver disease was not statistically significant (P=0.28).
The mean AST/ALT ratio for children with a history of formula feeding was 1.02±0.46, compared to 0.82±0.28 for those with no history of formula feeding. The medians were 0.87 for those with a history of formula feeding and 0.27 for those with no history of formula feeding, and their IQR were 0.57 and 0.78, respectively.
Overall, the AST/ALT ratio had a median of 0.39, an IQR of 0.78, and a mean of 0.89±0.45. The difference in the AST/ALT ratio based on the history of formula feeding was not statistically significant (P=0.37) (Table 1).
Biochemical markers
The fasting blood glucose level in children (n=48) showed a mean of 114.56±80.53 mg/dL, a median of 93 mg/dL, and an IQR of 20 mg/dL. The TG level in children (n=51) had a mean of 142.54±65.35 mg/dL, a median of 121 mg/dL, and an IQR of 80 mg/dL. The TC level in children (n=52) had a mean of 166.71±35 mg/dL, a median of 168 mg/dL, and an IQR of 41.5 mg/dL.
Discussion
NAFLD encompasses a spectrum of liver abnormalities whose prevalence is increasing [20]. The spectrum of this disease includes steatosis (fatty infiltration of the liver), steatohepatitis (inflammation and damage to liver cells), followed by liver fibrosis and, ultimately, cirrhosis [21]. The pathogenesis of NAFLD in children is multifactorial, involving a combination of genetic, environmental, and lifestyle factors [22]. Although the underlying pathophysiological mechanism of pediatric NAFLD is not fully understood, it is strongly associated with obesity and insulin resistance, as well as genetic predisposition [23]. In children, the NAFLD is usually associated with risk factors such as poor eating habits (especially high intake of sweets and fast foods), a sedentary lifestyle, and other metabolic factors [22, 23]. From a genetic perspective, new polymorphisms, such as PNPLA3, TM6SF2, MBOAT7, and GCKR, have also been identified and are used to predict the development and severity of NAFLD in both adults and children, where their interaction with environmental factors have been reported [24]. Changes in lifestyle patterns over the past few decades have resulted in increased obesity rates in the general population, including children and adolescents [25]. A systematic review reported a NAFLD prevalence of 27.88% in the pediatric population of Iran [26], which is considerable.
This study examined the indicators of NAFLD in children, including demographic and laboratory characteristics, screen time, PA level, and dietary habits. The results revealed higher levels in laboratory data, including ALT, TG, and TC in these patients, compared to their normal ranges for children. Also, girls had a significantly higher AST/ALT ratio than boys.
In this study, 74% of the children with NAFLD were male and 26% were female. Other studies with larger sample sizes have also supported our findings, indicating that boys, regardless of age and Tanner stage, have a higher prevalence of NAFLD compared to girls [27]. Consistent with our results, a systematic review and meta-analysis of 15 case-control studies involving 1,595 children (824 in the patient group and 771 in the healthy group) found that the BMI and waist circumference in the NAFLD group were significantly higher than in the control group [28].
The relationship between the history of formula feeding and NAFLD in childhood is a relatively new research topic. Some studies suggest that formula feeding, particularly due to its association with rapid weight gain, may be linked to an increased risk of NAFLD in children. Conversely, breastfeeding is associated with a reduced risk of NAFLD, likely due to its potential protective effects on metabolic health [29]. The association between NAFLD in children and the consumption of unhealthy foods is a significant concern in modern society, considering the rise in pediatric obesity and unhealthy dietary patterns [30]. Unhealthy foods contribute to the development and progression of NAFLD in children through various mechanisms such as high calorie intake, excessive consumption of sweets, eating foods high in fat and cholesterol, eating processed foods, and eating foods low in essential nutrients [31].
NAFLD is often asymptomatic, making it challenging to be diagnosed [6]. Due to the subtle nature of this disease, laboratory tests can provide valuable information for identifying patients at risk for NAFLD. It is the most common cause of abnormal liver function in children [32]. Currently, the ALT level is recommended as a biomarker of NAFLD [32]. The ALT level measurement is non-invasive and has acceptable sensitivity [32]. In our study, the mean values of lipid profile in children were in the abnormal range, which can be a potential risk factor, and the children with high lipid profiles referred to the clinics for further treatment should be suspected of NAFLD.
This study had some limitations. With a cross-sectional design, it is not possible to assess causality. Additionally, the unavailability and incompleteness of information in some patient records led to a reduced sample size. Another limitation was the lack of cooperation from some parents in providing data related to their children’s lifestyles.
Conclusion
Most of the children with NAFLD referred to the study clinic in Tehran were male. More than half of them had a family history of NAFLD. In these children, the elevation of ALT level was greater than that of the AST level, both exceeding the upper limit of normal. Also, the TG and TC levels in children with NAFLD were higher than normal, but their fasting blood glucose level was normal.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.CHMC.REC.1400.260). Informed consent was obtained from the parents of all children before the study, and the study objectives and procedures were thoroughly explained to them. Participation in the study did not incur any additional costs for the patients. All personal information was kept confidential and handled in accordance with the relevant ethical guidelines.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Authors contributions
Conceptualization: Amin Ghanbari and Parisa Rahmani; Data collection: Amin Ghanbari and Bahar Allahverdi; Statistical analysis: Bahar Allahverdi and Pejman Rohani; Supervision: Pejman Rohani and Parisa Rahmani; Writing the original draft: Amin Ghanbari and Mohammadreza Pazoki; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to express their gratitude to the children and their families who involved in this study, as well as the medical staff at CMC for their support and collaboration. The authors also appreciate the guidance of their research advisors. Lastly, they thank their colleagues for their encouragement and constructive feedback throughout this research.
References
Hepatic steatosis, as fatty liver disease, refers to the accumulation of excess fat, especially triglycerides, within liver cells or hepatocytes [1]. Metabolic dysfunction-associated fatty liver disease is a new term suggested in 2020 for non-alcoholic fatty liver disease (NAFLD) as a systemic metabolic dysfunction [2]. When hepatocytes with fat droplets become inflamed and damaged, healthy cells respond by replacing the tissue damaged by the accumulation of triglycerides with normal hepatic tissue to repair the damage [3]. If this damaging process persists, the liver’s capacity to produce an adequate amount of healthy tissue will decrease, and fibrous tissue will take its place [4].
The NAFLD is becoming more widespread in children and adolescents worldwide, making it the most prevalent chronic liver disease in this age group, affecting 3-20% of them [5, 6]. Individuals with NAFLD may have vague symptoms or be asymptomatic, complicating the diagnosis process [7]. The most reliable method for the diagnosis of NAFLD in adults is liver biopsy due to its ability to provide accurate information about the extent of liver damage and the presence of inflammation [8]. Because of its invasive nature and the possibility of side effects, it may be challenging to be performed on children. Ultrasound is an alternate diagnostic tool for this purpose in children, offering a non-invasive way to assess liver health and detect abnormalities [9].
The NAFLDs, especially non-alcoholic steatohepatitis and fibrosis, are associated not only with liver-related problems but also with an increased risk of cardiovascular diseases, type 2 diabetes, and mortality in adulthood [10, 11]. The presence of NAFLD in children is more challenging since there are currently no specific non-invasive indicators available for the detection of liver inflammation in clinical settings [12]. Due to its chronic nature, NAFLD can negatively affect children’s health beyond their physical aspects. Obese children with NAFLD experience a lower quality of life compared to obese children without the NAFLD [13]. Children with NAFLD have more severe physical and psychological impairments in comparison with their healthy counterparts [13, 14].
The occurrence of NAFLD is increasing due to changes in lifestyle, such as a rise in sedentary behaviors and the consumption of high-calorie foods, although it is also often not diagnosed or identified [15, 16]. Hence, it is essential to implement early detection strategies, specifically targeting children with a greater susceptibility to this disease [17]. Given the longer lifespan of children with NAFLD compared to those with fatal diseases, they are more likely to experience the challenges associated with this condition for a longer period [18]. Therefore, it is crucial to have efficient screening methods for children who are at a higher risk of experiencing NAFLD. Screening for NAFLD is recommended as it enables the identification of the condition prior to the development of irreversible and advanced liver damage [17]. Gaining knowledge about the characteristics and control of NAFLD in children might provide a way to intervene early and modify the course of the condition. There is a lack of studies on the basic findings of children with NAFLD. Early detection of this disease may halt its development and alleviate its enduring adverse effects on the child’s well-being. Therefore, this study aims to identify the clinical indicators of NAFLD for children to manage its progression effectively and prevent complications.
Materials and Methods
Study design and participants
This is a descriptive-analytical study with a cross-sectional design conducted on children diagnosed with NAFLD referred to Children’s Medical Center in Tehran, Iran. All children with NAFLD at the Pediatric Gastroenterology and Hepatology Clinic of this hospital during 2021-2022 were included in the study (n=66). The NAFLD was diagnosed using ultrasound reports from expert radiologists. Inclusion criteria were age at diagnosis ≤18 years, diagnosis of NAFLD based on ultrasound or liver biopsy, elevation of alanine aminotransferase (ALT) level more than 1.5 times the upper limit of normal in obese or overweight children. Exclusion criteria were insufficient imaging evidence of NAFLD, presence of viral hepatitis, autoimmune hepatitis, Wilson’s disease, metabolic diseases, or genetic disorders leading to hepatic steatosis (e.g. glycogen storage disease), and presence of fatty liver due to alcohol consumption or use of steatogenic drugs.
Data collection
Demographic and laboratory data of patients were collected from the SABARA system (an electronic health record system used in Iran) using a checklist designed by the authors. The extracted data included patients’ demographic and laboratory data (age, gender, weight, body mass index [BMI], family history of fatty liver disease, fasting blood glucose level, serum ALT and aspartate transaminase [AST] levels, serum triglyceride [TG] level, total cholesterol [TC] level, history of formula feeding during infancy, history of breastfeeding during infancy), physicians’ prescriptions, and related factors. Information regarding patients’ lifestyles, including frequency of physical activity (PA) per week, frequency of eating fast food per week, and frequency of eating sweet foods per week, was gathered using a standard nutritional screening questionnaire for adolescents developed by the Iranian Ministry of Health and Medical Education in 2016 [19]. Based on this questionnaire, the PA level was classified into three categories: Very little or no purposeful PA, <420 minutes per week, and ≥420 minutes per week. Also, the frequency of eating fast foods and sweets was classified into three categories: Almost daily, weekly (once or twice per week), and rarely/never (once or twice per month). Accordingly, the daily screen time was classified into three categories: >2 hours per day, approximately 2 hours per day, and <2 hours per day.
Statistical analysis
Qualitative data were reported using frequency, while quantitative data were reported using the Mean±SD (if they had a normal distribution) and median and interquartile range (IQR) (if they had no normal distribution). If the data distribution was normal, to examine the difference between categorical variables, the chi-square test and ANOVA were used, followed by Fisher’s exact test, if necessary. To examine the difference between quantitative variables with binary qualitative variables, the independent sample t-test was used. In cases of abnormal data distribution, the equivalent non-parametric tests were used. All statistical analyses were performed in SPSS software, version 27. The significance level was set at 0.05.
Results
Participants’ demographic characteristics, weight and BMI
In this study, 66 children with NAFLD participated. The majority of them were boys (74.0%, n=49). Their mean age was 118.04±42.01 months, ranged 8-196 months. The mean weight and BMI were 57.37±28.4 kg and 26.37±7.41 kg/m2, respectively (Figure 1). Most of the children had a positive family history of fatty liver disease (57.4%, n=31 out of 54) and had a history of using infant formula before 6 months (24.1%, n=13 out of 54).
Participants’ PA, dietary habits, and screen time
Based on the nutritional screening tool, 19 children (35.19%) were classified in the first group (very little or no purposeful PA), 27 children (50%) in the second group (<420 minutes per week), and 8 children (14.81%) in the third group (≥420 minutes per week). Additionally, based on gender, 29.27% (n=12) of boys and 53.85% of girls (n=7) were in the first group; 53.66% of boys (n=22) and 38.46% of girls (n=5) were in the second group, and 17.07% of boys (n=7) and 7.69% of girls (n=1) were in the third group (Figure 2). Regarding the frequency of eating sweet foods, according to the parents of children, 37 children (68.52%) were classified in the first group (almost daily), 7(12.96%) in the second group (weekly), and 10(18.52%) in the third group (rarely/never). Regarding the frequency of eating fast foods, 2 children (3.7%) were classified in the first group, 12(12.22%) in the second group, and 40(74.07%) in the third group. Overall, 37 children (68.52%) were classified in the first group, 7(12.96%) in the second group, and 10(18.52%) in the third group. Regarding the daily screen time, according to the parents of children, 35 children (64.81%) fell into the first group (>2 hours per day), 11 individuals (20.37%) into the second group (2 hours per day), and 8 individuals (14.81%) into the third group (<2 hours per day) (Figure 3).
Participants’ laboratory characteristics
AST and ALT Levels
The mean AST level for males was 37.28±15.71 U/L, and for females was 52.11±59.81 U/L. The median AST levels for males and females were 35 U/L and 34 U/L, respectively. The IQR for males and females was 22 and 26, respectively. Overall, the mean AST level in children was 41.55±34.74 U/L, with a median of 34 U/L and an IQR of 24. The difference in the AST level between males and females was not statistically significant (P=0.43).
Males had a mean ALT level of 57.69±40.46 U/L, whereas females had a higher mean level (62.52±96.34 U/L). The median ALT levels for males and females were 49 U/L and 32 U/L, respectively. The IQR of ALT was 41 in males and 48.50 in females. Overall, the mean ALT level was 59.06±60.72 U/L with a median of 45.5 U/L and an IQR of 44.5. The P for the difference in ALT levels between genders was 0.12, indicating no significant difference.
AST/ALT ratio
The AST/ALT ratio had a mean of 0.81±0.36 for males and 1.08±0.4 for females. The medians were 0.74 for males and 0.45 for females, with IQRs of 0.356 and 1.06, respectively. There was a significant difference in the AST/ALT ratio between males and females (P=0.01).
Participants with a family history of fatty liver disease had a mean AST/ALT ratio of 0.83±0.34, while those with no family history had a mean of 0.95±0.34. The medians were 0.78 for those with a family history and 0.43 for those with no family history, and their corresponding IQR were 0.28 and 0.92, respectively. The difference in the AST/ALT ratio based on the family history of fatty liver disease was not statistically significant (P=0.28).
The mean AST/ALT ratio for children with a history of formula feeding was 1.02±0.46, compared to 0.82±0.28 for those with no history of formula feeding. The medians were 0.87 for those with a history of formula feeding and 0.27 for those with no history of formula feeding, and their IQR were 0.57 and 0.78, respectively.
Overall, the AST/ALT ratio had a median of 0.39, an IQR of 0.78, and a mean of 0.89±0.45. The difference in the AST/ALT ratio based on the history of formula feeding was not statistically significant (P=0.37) (Table 1).

Biochemical markers
The fasting blood glucose level in children (n=48) showed a mean of 114.56±80.53 mg/dL, a median of 93 mg/dL, and an IQR of 20 mg/dL. The TG level in children (n=51) had a mean of 142.54±65.35 mg/dL, a median of 121 mg/dL, and an IQR of 80 mg/dL. The TC level in children (n=52) had a mean of 166.71±35 mg/dL, a median of 168 mg/dL, and an IQR of 41.5 mg/dL.
Discussion
NAFLD encompasses a spectrum of liver abnormalities whose prevalence is increasing [20]. The spectrum of this disease includes steatosis (fatty infiltration of the liver), steatohepatitis (inflammation and damage to liver cells), followed by liver fibrosis and, ultimately, cirrhosis [21]. The pathogenesis of NAFLD in children is multifactorial, involving a combination of genetic, environmental, and lifestyle factors [22]. Although the underlying pathophysiological mechanism of pediatric NAFLD is not fully understood, it is strongly associated with obesity and insulin resistance, as well as genetic predisposition [23]. In children, the NAFLD is usually associated with risk factors such as poor eating habits (especially high intake of sweets and fast foods), a sedentary lifestyle, and other metabolic factors [22, 23]. From a genetic perspective, new polymorphisms, such as PNPLA3, TM6SF2, MBOAT7, and GCKR, have also been identified and are used to predict the development and severity of NAFLD in both adults and children, where their interaction with environmental factors have been reported [24]. Changes in lifestyle patterns over the past few decades have resulted in increased obesity rates in the general population, including children and adolescents [25]. A systematic review reported a NAFLD prevalence of 27.88% in the pediatric population of Iran [26], which is considerable.
This study examined the indicators of NAFLD in children, including demographic and laboratory characteristics, screen time, PA level, and dietary habits. The results revealed higher levels in laboratory data, including ALT, TG, and TC in these patients, compared to their normal ranges for children. Also, girls had a significantly higher AST/ALT ratio than boys.
In this study, 74% of the children with NAFLD were male and 26% were female. Other studies with larger sample sizes have also supported our findings, indicating that boys, regardless of age and Tanner stage, have a higher prevalence of NAFLD compared to girls [27]. Consistent with our results, a systematic review and meta-analysis of 15 case-control studies involving 1,595 children (824 in the patient group and 771 in the healthy group) found that the BMI and waist circumference in the NAFLD group were significantly higher than in the control group [28].
The relationship between the history of formula feeding and NAFLD in childhood is a relatively new research topic. Some studies suggest that formula feeding, particularly due to its association with rapid weight gain, may be linked to an increased risk of NAFLD in children. Conversely, breastfeeding is associated with a reduced risk of NAFLD, likely due to its potential protective effects on metabolic health [29]. The association between NAFLD in children and the consumption of unhealthy foods is a significant concern in modern society, considering the rise in pediatric obesity and unhealthy dietary patterns [30]. Unhealthy foods contribute to the development and progression of NAFLD in children through various mechanisms such as high calorie intake, excessive consumption of sweets, eating foods high in fat and cholesterol, eating processed foods, and eating foods low in essential nutrients [31].
NAFLD is often asymptomatic, making it challenging to be diagnosed [6]. Due to the subtle nature of this disease, laboratory tests can provide valuable information for identifying patients at risk for NAFLD. It is the most common cause of abnormal liver function in children [32]. Currently, the ALT level is recommended as a biomarker of NAFLD [32]. The ALT level measurement is non-invasive and has acceptable sensitivity [32]. In our study, the mean values of lipid profile in children were in the abnormal range, which can be a potential risk factor, and the children with high lipid profiles referred to the clinics for further treatment should be suspected of NAFLD.
This study had some limitations. With a cross-sectional design, it is not possible to assess causality. Additionally, the unavailability and incompleteness of information in some patient records led to a reduced sample size. Another limitation was the lack of cooperation from some parents in providing data related to their children’s lifestyles.
Conclusion
Most of the children with NAFLD referred to the study clinic in Tehran were male. More than half of them had a family history of NAFLD. In these children, the elevation of ALT level was greater than that of the AST level, both exceeding the upper limit of normal. Also, the TG and TC levels in children with NAFLD were higher than normal, but their fasting blood glucose level was normal.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (Code: IR.TUMS.CHMC.REC.1400.260). Informed consent was obtained from the parents of all children before the study, and the study objectives and procedures were thoroughly explained to them. Participation in the study did not incur any additional costs for the patients. All personal information was kept confidential and handled in accordance with the relevant ethical guidelines.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Authors contributions
Conceptualization: Amin Ghanbari and Parisa Rahmani; Data collection: Amin Ghanbari and Bahar Allahverdi; Statistical analysis: Bahar Allahverdi and Pejman Rohani; Supervision: Pejman Rohani and Parisa Rahmani; Writing the original draft: Amin Ghanbari and Mohammadreza Pazoki; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to express their gratitude to the children and their families who involved in this study, as well as the medical staff at CMC for their support and collaboration. The authors also appreciate the guidance of their research advisors. Lastly, they thank their colleagues for their encouragement and constructive feedback throughout this research.
References
- Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. Journal of Gastroenterology. 2013; 48(4):434-41. [DOI:10.1007/s00535-013-0758-5] [PMID]
- Gofton C, Upendran Y, Zheng MH, George J. MAFLD: How is it different from NAFLD? Clinical and Molecular Hepatology. 2023; 29(Suppl):S17-31. [DOI:10.3350/cmh.2022.0367] [PMID]
- Chávez-Tapia NC, Rosso N, Uribe M, Bojalil R, Tiribelli C. Kinetics of the inflammatory response induced by free fatty acid accumulation in hepatocytes. Annals of Hepatology. 2014; 13(1):113-20. [DOI:10.1016/S1665-2681(19)30912-3]
- Cordero-Espinoza L, Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. Journal of Clinical Investigation. 2018; 128(1):85-96. [DOI:10.1172/JCI93562] [PMID]
- Temple JL, Cordero P, Li J, Nguyen V, Oben JA. A guide to non-alcoholic fatty liver disease in childhood and adolescence. International Journal of Molecular Sciences. 2016; 17(6):947. [DOI:10.3390/ijms17060947] [PMID]
- Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. 2016; 150(8):1798-810. [DOI:10.1053/j.gastro.2016.03.009] [PMID]
- Hashimoto E, Tokushige K, Ludwig J. Diagnosis and classification of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Current concepts and remaining challenges. Hepatology Research. 2015; 45(1):20-8. [DOI:10.1111/hepr.12333] [PMID]
- Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clinic Proceedings. 2015; 90(9):1233-46. [DOI:10.1016/j.mayocp.2015.06.013] [PMID]
- Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents. Journal of Pediatric Gastroenterology and Nutrition. 2012; 54(5):700-13. [DOI:10.1097/MPG.0b013e318252a13f] [PMID]
- Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Current Diabetes Reports. 2021; 21(5):15. [DOI:10.1007/s11892-021-01383-7] [PMID]
- Morrison AE, Zaccardi F, Khunti K, Davies MJ. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver International. 2019; 39(3):557-67. [DOI:10.1111/liv.13994] [PMID]
- Fahoum K, Ying X, Magahis PT, Ross J, Basu E, Shen NT, et al. Non-invasive markers of inflammation in alcohol-associated liver disease: A scoping review. Journal of Gastroenterology and Hepatology. 2024; 39(2):245-55. [DOI:10.1111/jgh.16432] [PMID]
- Kerkar N, D'Urso C, Van Nostrand K, Kochin I, Gault A, Suchy F, et al. Psychosocial outcomes for children with nonalcoholic fatty liver disease over time and compared with obese controls. Journal of Pediatric Gastroenterology and Nutrition. 2013; 56(1):77-82. [DOI:10.1097/MPG.0b013e31826f2b8c] [PMID]
- Karaivazoglou K, Kalogeropoulou M, Assimakopoulos S, Triantos C. Psychosocial issues in pediatric nonalcoholic fatty liver disease. Psychosomatics. 2019; 60(1):10-7. [DOI:10.1016/j.psym.2018.09.001] [PMID]
- DiStefano JK, Shaibi GQ. The relationship between excessive dietary fructose consumption and paediatric fatty liver disease. Pediatric Obesity. 2021; 16(6):e12759. [DOI:10.1111/ijpo.12759] [PMID]
- Saklayen MG. The global epidemic of the metabolic syndrome. Current Hypertension Reports. 2018; 20(2):12. [DOI:10.1007/s11906-018-0812-z] [PMID]
- Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nature Reviews Gastroenterology & Hepatology. 2019; 16(9):517-30. [DOI:10.1038/s41575-019-0169-z] [PMID]
- Goldner D, Lavine JE. Nonalcoholic fatty liver disease in children: Unique considerations and challenges. Gastroenterology. 2020; 158(7):1967-83.e1. [DOI:10.1053/j.gastro.2020.01.048] [PMID]
- Abdullahi Z, Torabi P, Salehi Mazandarani F, Sadeghi Qutbabadi F, Minaei M, Nobakht Haghighi F, et al. [Nutritional Care and Services Complex In the Health System Transformation Program in the Special Health Care Area, Health Care Provider, Nutritionist, and Physician (Persian)]. Tehran: Office of Community Nutrition Improvement; 2016. [Link]
- Kim HJ. Significance of triglyceride-to-high-density lipoprotein cholesterol ratio in children with non-alcoholic fatty liver disease. Pediatric Gastroenterology, Hepatology & Nutrition. 2023; 26(6):312-9 [DOI:10.5223/pghn.2023.26.6.312] [PMID]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nature Reviews Gastroenterology & Hepatology. 2013; 10(11):686-90. [DOI:10.1038/nrgastro.2013.171] [PMID]
- Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model.” World Journal of Gastroenterology. 2018; 24(27):2974-83. [DOI:10.3748/wjg.v24.i27.2974] [PMID]
- Peng L, Wu S, Zhou N, Zhu S, Liu Q, Li X. Clinical characteristics and risk factors of nonalcoholic fatty liver disease in children with obesity. BMC Pediatrics. 2021; 21(1):122. [DOI:10.1186/s12887-021-02595-2] [PMID]
- Lin YC, Wu CC, Ni YH. New perspectives on genetic prediction for pediatric metabolic associated fatty liver disease. Front Pediatr. 2020; 8:603654. [DOI: 10.3389/fped.2020.603654] [PMID]
- Jebeile H, Kelly AS, O’Malley G, Baur LA. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. The lancet. Diabetes & Endocrinology. 2022; 10(5):351-65. [DOI:10.1016/S2213-8587(22)00047-X] [PMID]
- Salehisahlabadi A, Jadid H. [The prevalence of non-alcoholic fatty liver disease in Iranian children and adolescents: A systematic review and meta-analysis (Persian)]. Journal of Sabzevar University of Medical Sciences. 2018; 25(4):487-94. [Link]
- Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology. 2019; 70(4):1457-69. [DOI:10.1002/hep.30626] [PMID]
- He Y, Cao L, Zhou C, Zhang R, Zeng M, Peng X, et al. Relationship between obesity related indicators and non-alcoholic fatty liver disease in children: A systematic review and meta-analysis. Translational Pediatrics. 2023; 12(3):429-44. [DOI:10.21037/tp-23-123] [PMID]
- Ayonrinde OT, Oddy WH, Adams LA, Mori TA, Beilin LJ, de Klerk N, et al. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. Journal of Hepatology. 2017; 67(3):568-76. [DOI:10.1016/j.jhep.2017.03.029] [PMID]
- Alisi A, Feldstein AE, Villani A, Raponi M, Nobili V. Pediatric nonalcoholic fatty liver disease: A multidisciplinary approach. Nature Reviews. Gastroenterology & Hepatology. 2012; 9(3):152-61. [DOI:10.1038/nrgastro.2011.273] [PMID]
- Bansal M, Vohra R, Sood A, Bhardwaj P. Correlation between metabolic, liver profile, dietary habits and ultrasound scan determined non-alcoholic fatty liver disease changes in children aged 6- 18 years with body mass index. Sri Lanka Journal of Child Health. 2018; 47(2):125-8. [DOI:10.4038/sljch.v47i2.8477]
- Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic fatty liver disease in children. Seminars Liver Disease. 2018; 38(1):1-13. [DOI:10.1055/s-0038-1627456] [PMID]
Type of Study: Original Article |
Subject:
Pediatrics
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |