Volume 13, Issue 1 (Winter 2025)
Iran J Health Sci 2025, 13(1): 65-74 |
Back to browse issues page
Ethics code: 0
Clinical trials code: 0
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hosseini M, Vaheb S, Azadvari M, Rastkar M, Ghajarzadeh M, Cheraghali M et al . The Effects of OnabotulinumtoxinA Treatment for Spasticity in Patients With Spinal Cord Injury: A Systematic Review and Meta-analysis. Iran J Health Sci 2025; 13 (1) :65-74
URL: http://jhs.mazums.ac.ir/article-1-942-en.html
URL: http://jhs.mazums.ac.ir/article-1-942-en.html
Maryam Hosseini 

 , Saeed Vaheb
, Saeed Vaheb 

 , Mohaddeseh Azadvari
, Mohaddeseh Azadvari 

 , Mohsen Rastkar
, Mohsen Rastkar 

 , Mahsa Ghajarzadeh
, Mahsa Ghajarzadeh 

 , Maryam Cheraghali
, Maryam Cheraghali 

 , Seyede Zahra Emami Razavi *
, Seyede Zahra Emami Razavi * 




 , Saeed Vaheb
, Saeed Vaheb 

 , Mohaddeseh Azadvari
, Mohaddeseh Azadvari 

 , Mohsen Rastkar
, Mohsen Rastkar 

 , Mahsa Ghajarzadeh
, Mahsa Ghajarzadeh 

 , Maryam Cheraghali
, Maryam Cheraghali 

 , Seyede Zahra Emami Razavi *
, Seyede Zahra Emami Razavi * 


Joint Reconstruction Research Center, Tehran University of Medical Sciences, Tehran, Iran. , zemamirazavi@gmail.com
Full-Text [PDF 860 kb]
(357 Downloads)
| Abstract (HTML) (906 Views)
Full-Text: (1586 Views)
Introduction
pinal cord injury (SCI) is a life-altering condition predominantly affecting young individuals and men [1]. This condition not only causes physical impairment but also significantly impacts the psychological, social, and marital status of patients, ultimately interfering with daily activities and diminishing quality of life [2, 3]. Among the most common complications of SCI is spasticity, with a prevalence ranging between 31% and 78% of patients [4, 5]. Spasticity is characterized by intermittent or sustained involuntary muscle activation, which can affect joints such as the shoulders, elbows, wrists, ankles, knees, and hips, leading to abnormal postures and limb pain [5].
Spasticity limits patients’ ability to perform self-care, disrupts sleep, and causes pain, fatigue, and a negative body image, all contributing to a reduced quality of life [4, 5]. The mechanism behind spasticity in SCI arises from disruption in the inhibitory motor pathways, leading to elevated muscle tone and reflexes [4]. In managing spasticity, it is crucial to consider factors like the length of time since injury, the severity of symptoms, patient support systems, and the patient’s and caregivers’ preferences [5, 6]. Oral medications such as baclofen, diazepam, and clonidine are frequently used; however, these therapies often fail to achieve full symptom relief [7].
Botulinum toxin type A (BOTOX®), a neurotoxin produced by Clostridium botulinum, is an approved treatment for spasticity in both upper and lower limbs [5]. This treatment typically benefits 100 to 150 days; patients usually require reinjections to maintain its effects [7].
Previous studies have reported improvement in spasticity following onabotulinumtoxinA injections in SCI patients. However, a systematic review of these studies is needed to synthesize the available evidence and assess the overall efficacy of this treatment.
This study aims to systematically review and evaluate the effects of onabotulinumtoxinA on spasticity in individuals with SCI. The review will also assess the quality of the included studies using predefined criteria. Specifically, the preferred reporting items for systematic reviews and meta-analysis (PRISMA) or the Cochrane risk of bias tool will be employed to evaluate study design, sample size, blinding, and statistical methods. The risk of bias will be reported and discussed in detail to provide transparency regarding the strength of the evidence.
Moreover, although the lack of a meta-analysis is a limitation of this study, the heterogeneity in study designs and outcome measures precluded performing a combined analysis. Future research should aim to standardize outcome measures to facilitate meta-analyses and provide more robust estimates of treatment effects.
Materials and Methods
Search strategy
We conducted a systematic review following the PRISMA guidelines. A comprehensive search of multiple databases, including PubMed, Scopus, EMBASE, Web of Science, and Google Scholar, was performed to identify relevant studies on spasticity treatment in SCI published until September 2023. We also searched gray literature, such as conference abstracts and reference lists of included studies, to ensure a thorough review of all available evidence. The search strategy was designed to cover various studies on pharmacological and non-pharmacological treatments for spasticity, ensuring comprehensive coverage of existing research.
While the Cochrane Library is also a valuable resource, we chose not to include it because it primarily focused on peer-reviewed articles and clinical trials indexed in the broader biomedical databases mentioned. Cochrane reviews primarily aggregate existing systematic reviews, and our objective was to focus on primary research studies that evaluated the efficacy of onabotulinumtoxinA in treating spasticity in SCI patients.
However, the inclusion of Cochrane could be considered in future review updates to ensure an even more comprehensive search strategy.
The MeSH terms that were used for searching in PubMed were as follows:
((“Spinal cord injur*”) OR ((“cord trauma*”) AND (spinal)) OR (“spinal cord trauma*”) OR ((trauma*) AND (“spinal cord”)) OR ((myelopath*) AND (traumatic)) OR (“traumatic myelopath*”) OR ((injur*) AND (spinal cord)) OR ((“cord injur*) AND (spinal)) OR (“spinal cord transection*”) OR ((“cord transection*”) AND (spinal)) OR ((Transection*) AND (“spinal cord”)) OR (“spinal cord laceration*”) OR ((“cord laceration*) AND (spinal)) OR ((laceration*) AND (“spinal cord”)) OR (“post-traumatic myelopath*”) OR ((myelopath*) AND (post-traumatic)) OR (“post traumatic myelopath*”) OR ((contusion*) AND (“spinal cord”)) OR ((“cord contusion*”) AND (spinal)) OR (“spinal cord contusion*”)) AND (((“botulinum toxin*”) AND (“type A”)) OR (“clostridium botulinum A toxin”) OR (“botulinum toxin A”) OR ((“toxin A”) AND (botulinum)) OR (“botulinum neurotoxin A”) OR ((“neurotoxin A”) AND (botulinum)) OR (“botulinum A toxin”) OR ((toxin) AND (“botulinum A”)) OR (“botulinum toxin type A”) OR (“botulinum neurotoxin type A”) OR (“Clostridium botulinum toxin type A”) OR (meditoxin) OR (Botox) OR (neuronox) OR (oculinum) OR (vistabex) OR (onabotulinumtoxina) OR (“onabotulinumtoxin A”) OR (Vistabel) OR (“botulinum toxin*”) OR (“botulinum neurotoxin*”) OR ((toxin*) AND (botulinum)) OR (“clostridium botulinum toxin*”) OR ((toxin*) AND (“Clostridium botulinum”)) OR (“botulinum neurotoxin*”) OR ((neurotoxin*) AND (botulinum)) OR (botulin)).
Inclusion criteria
We included randomized controlled trials (RCTs) or cohort studies that evaluated the efficacy of onabotulinumtoxinA (Botox) in treating spasticity in patients with spinal cord injury.
Exclusion criteria
Studies such as letters to the editor, case-control studies, case reports, and cross-sectional studies were excluded. Cohort studies without a control group were also excluded.
Data extraction
Two independent researchers performed the search, and duplicates were removed before screening the titles and abstracts. In cases of discrepancy, a third researcher was consulted to resolve disagreements. After carefully evaluating the full texts of potentially relevant articles, data were extracted and recorded into data sheets.
Data regarding the total number of participants, first author, publication year, country of origin, mean age, follow-up time, the site of injection, main findings, and side effects were extracted and recorded.
Risk of bias assessment
We assessed the risk of bias in the included studies using two established tools: The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials [8]and the Newcastle-Ottawa scale (NOS) for cohort studies [9].
Cochrane tool: This tool evaluates several specific domains of bias, including:
Selection bias: Assessing how participants were selected and whether the randomization process was adequate.
Performance bias: Examining whether participants and personnel were blinded to the intervention.
Detection bias: Considering if the outcome assessors were blinded to the treatment allocation.
Attrition bias: Evaluating whether there were differences in dropout rates between groups and if the reasons for dropout were reported.
Reporting bias: Analyzing whether the study reported all prespecified outcomes and if any were omitted.
NOS: This scale is used for cohort studies and assesses three domains:
Selection: Evaluating how representative the cohort is and the adequacy of the selection of exposed and non-exposed cohorts.
Comparability: Assessing the comparability of cohorts based on the design or analysis.
Outcome: Considering the assessment of outcomes and whether they were adequately assessed.
The results of the risk of bias assessments will be summarized and discussed in the results section, providing a clear overview of the methodological quality of the included studies and their implications for the findings of this systematic review.
Statistical analysis
No specific statistical analysis was conducted, as this study was designed as a systematic review. However, a meta-analysis could have been performed if the included studies were sufficiently homogeneous regarding study design, interventions, and outcome measures. Unfortunately, conducting a meta-analysis was not feasible due to the significant heterogeneity among the studies, including differences in injection sites, dosing regimens, follow-up periods, and spasticity assessment tools.
It would be beneficial to standardize outcome measures and study designs in future studies to facilitate meta-analyses. Such efforts would allow for more robust quantitative estimates of the effects of onabotulinumtoxinA on spasticity in SCI patients.
Results
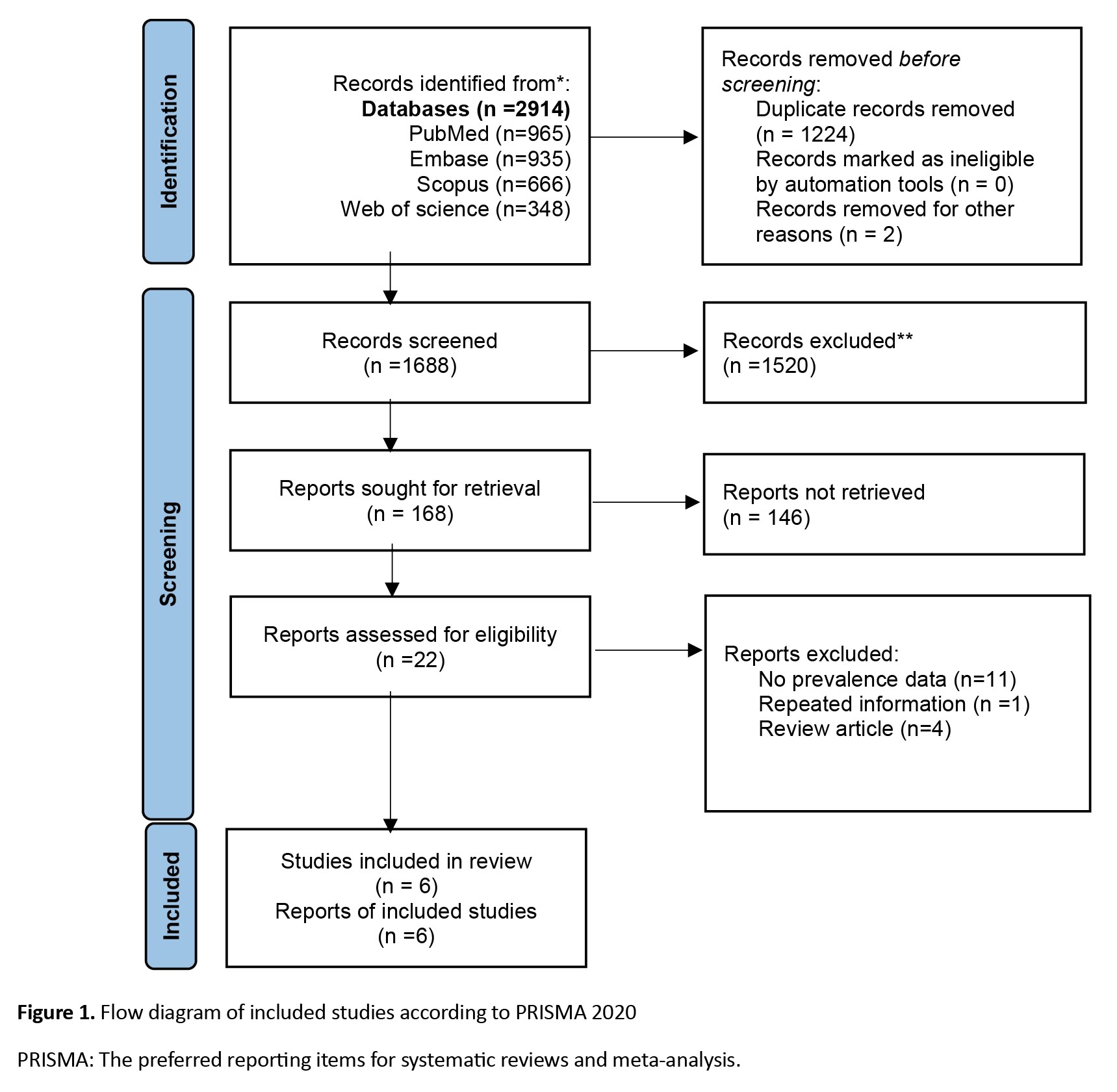
A literature search revealed 2914 articles; after deleting duplicates, 1688 remained. After careful evaluation of the full texts, six articles remained (Figure 1). Two studies were from the USA, and the most common site of injection was the upper limbs. The mean age ranged between 36 and 50 years, and the number of patients was between 9 and 112 (Table 1).
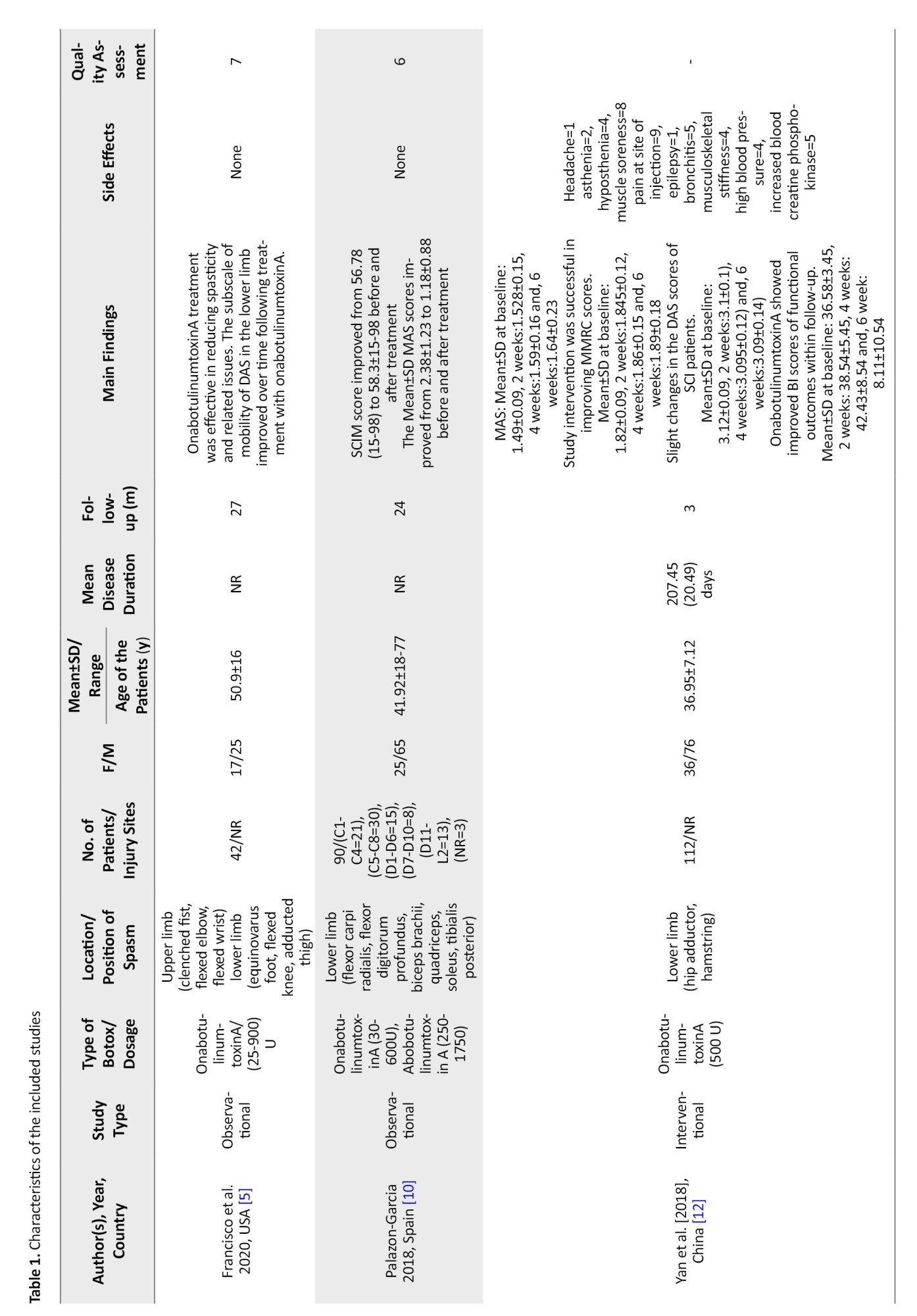
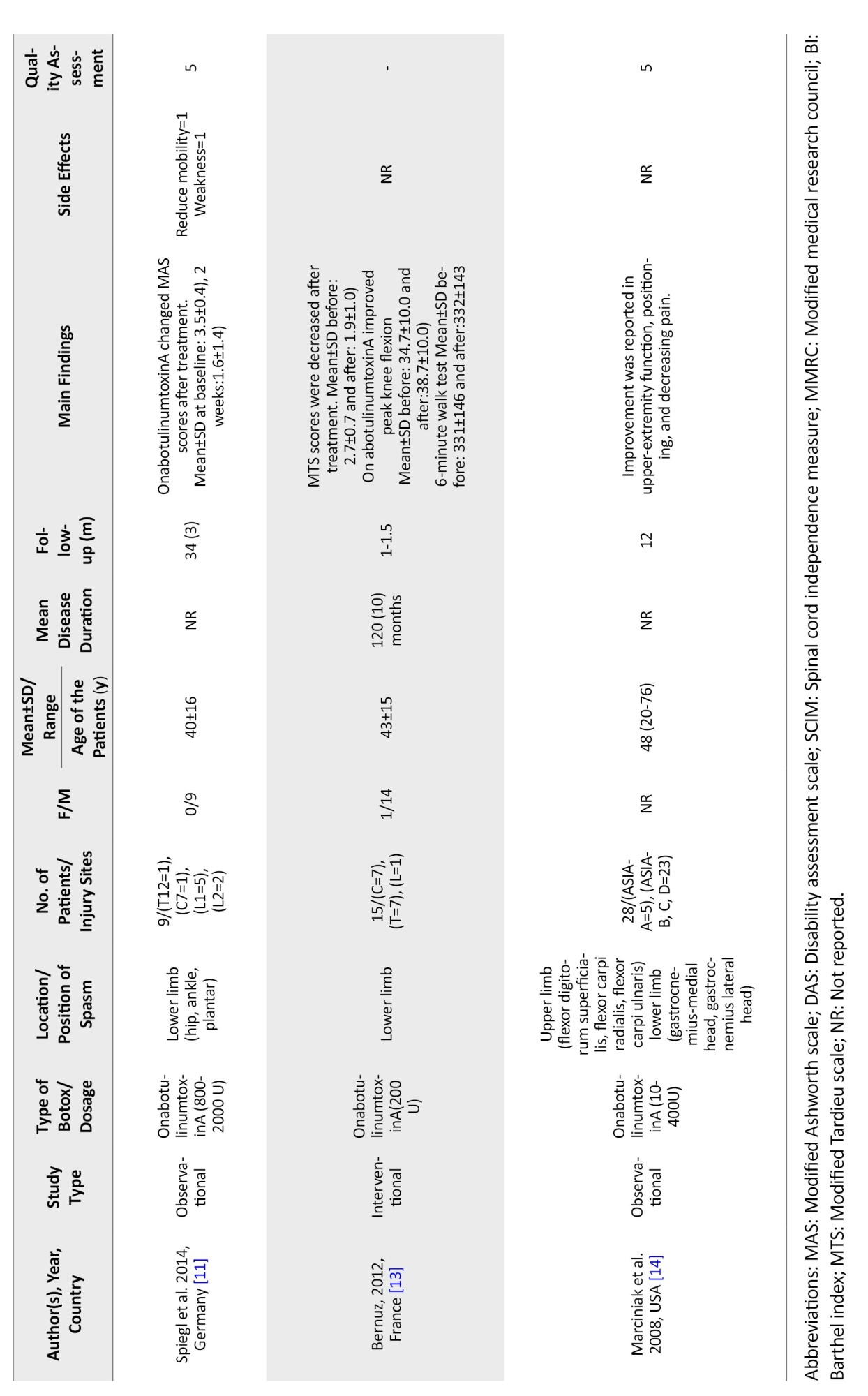
In the study by Francisco et al., the patients showed improvement in spasticity and spasticity-related pain after treatment with onabotulinumtoxinA [5]. At the same time, Palazon-Garcia [10] and Spiegl [11] reported improvement in the modified Ashworth scale (MAS) after treatment, which was not in line with the findings of Yan et al. [12]. A decrease in modified Tardieu scale (MTS) and peak knee flexion was reported by Bernuz et al. [13] (Table 1).
Marciniak et al. found improvement in upper-extremity function, positioning, and pain after treatment [14].
Discussion
Spasticity is a disabling complication in patients with SCI. Most published clinical trials regarding the effects of Botox injection on spasticity included patients with stroke or cerebral palsy [15, 16]. This outcome introduces important questions regarding the applicability of the findings to the SCI population. While the pathophysiology of spasticity may share similarities across these conditions, differences in the underlying mechanisms, injury profiles, and patient characteristics can significantly impact treatment responses. Therefore, caution should be exercised when generalizing results from studies focusing on stroke or cerebral palsy patients to those with SCI.
Food and Drug Administration (FDA) approved the administration of Botox for detrusor hyperactivity in patients with SCI in 2011 [13]. As spasticity is generalized in patients with SCI, administration of Botox in muscle groups that dominate the spasticity profile could decrease pain and discomfort related to spasticity [5].
Palazon-Garcia et al. [10] enrolled 90 patients with SCI, while more than 50% received Botox in their lower extremities. Their results showed that Botox injection improved all spasticity-related variables (tone, articular limitations, pain). Specifically, they noted reductions in the MAS scores, indicating decreased muscle tone and improvements in functional mobility assessed through standardized tests. These improvements were noted to last for approximately 12 to 16 weeks following injection. More improvement was observed in patients with incomplete injury and American SCI Association (ASIA) impairment scale D as the administration of Botox was focal in these cases. These findings align with previous research indicating that onabotulinumtoxinA is effective in managing spasticity, particularly in lower extremities, and suggest that this treatment may enhance the overall quality of life for individuals with SCI patients with focal spasticity better improvement [10].
Marciniak et al. enrolled 28 patients with SCI and administered Botox in most flexors of the upper extremities and antigravity muscles of the lower extremities. They reported improvement in ambulation, positioning, function of the upper extremity, and pain. They also found that early administration of Botox vs late administration did not affect the effectiveness [14].
By enrolling 336 patients with SCI in a clinical trial (evaluating baclofen, Botox, and only physical therapy), Yan et al. reported significant improvement in the disability assessment scale (DAS) in the baclofen group compared to the Botox group. This finding highlights the superior efficacy of baclofen in improving functional outcomes, though Botox remains a valuable option for targeted muscle spasticity management [12] (Table 2).
In a study conducted in Germany, Spiegl et al. [11]enrolled 9 patients with traumatic SCI and followed them up for 2 years. The maximum dose of Botox injection administered was 2000 units, and the results were deemed satisfactory in 6 cases. The therapeutic effects of Botox lasted, on average, for approximately 9 months, demonstrating the potential for sustained relief in a subset of patients [11]. However, the small sample size and variability in outcomes suggest a need for further investigation to identify patient-specific factors that may influence the efficacy and duration of Botox treatment.
Spasticity is a common complication following SCI, with 26%-67% of patients developing spasticity during the acute phase. Over time, spasticity becomes even more prevalent, affecting between 46% and 78% of patients after one year [5]. This high incidence makes effective management critical, as spasticity can interfere with daily activities, sleep, social interactions, and overall quality of life. Patients typically seek treatment for spasticity when these symptoms significantly impact their functioning, underscoring the need for timely and effective interventions. Combination therapy, including oral medications, positioning, stretching, blocks, or intrathecal administration of medications, is recommended [6]. Patients with incomplete SCI may have worse experience of spasticity. For patients with focal spasticity, local administration of Botox using injection localization methods such as electromyography (EMG), electrical stimulation (E-stim), and ultrasonography is recommended [7]. The primary effect of Botox is on the neuro-muscular junction, and it also affects the sensory feedback loop [7]. Patients will benefit from decreased pain sensation by improving spasticity and inhibiting the release of P substance [6]. The dosage differs based on the patient’s weight, muscle size, and spasticity degree.
The current management of spasticity involves a combination of therapeutic approaches, including oral medications (such as baclofen or tizanidine), physical positioning and stretching, nerve blocks, or intrathecal administration of medications for more severe cases [6, 7]. It is noteworthy that patients with incomplete SCI may experience more severe and persistent spasticity, making management more challenging.
For focal spasticity, where specific muscle groups are more affected, local administration of botulinum toxin (Botox) is recommended. Injection localization methods, such as EMG, E-stim, and ultrasonography, are critical for ensuring the precise delivery of the toxin to the affected muscles. These techniques improve the accuracy of injections, optimizing the therapeutic effects of Botox and minimizing the risk of complications or suboptimal outcomes [10]. Standardizing these localization methods could enhance treatment efficacy and provide more reliable results for patients with focal spasticity.
One of the rare complications of Botox injection is generalized muscle weakness, which should be considered.
Conclusion
In conclusion, onabotulinumtoxinA appears to be an effective and well-tolerated treatment option for managing spasticity in individuals with spinal cord injury. This systematic review provides compelling evidence for its efficacy in reducing muscle tone and improving function. Given the complexities associated with spasticity in SCI, healthcare providers must adopt a multidisciplinary approach when considering treatment options, incorporating pharmacological, rehabilitative, and supportive strategies tailored to individual patient needs.
Future research should prioritize the standardization of outcome measures to facilitate meta-analyses and the accumulation of more robust evidence regarding the long-term effects of onabotulinumtoxinA in this population. Additionally, larger-scale studies are necessary to explore further this intervention’s efficacy and safety across diverse cohorts of SCI patients, ensuring that treatment modalities are optimized for improving quality of life.
Study limitations
This study has several limitations that should be acknowledged. First, the number of included studies was limited, which restricts the generalizability of the findings to the broader population of patients with SCI. A more extensive range of studies would provide a clearer understanding of the efficacy and safety of onabotulinumtoxinA (Botox) in this context.
Second, the included studies exhibited significant variability in their outcome measures and evaluation methodologies. This inconsistency complicates comparing results across studies and synthesizing data, which may lead to ambiguous conclusions about the treatment’s overall effectiveness.
Moreover, many studies had small sample sizes, which can contribute to variability in findings and may not adequately represent the diverse characteristics of the SCI population. Small sample sizes also limit the power of statistical analyses, potentially obscuring meaningful effects.
Furthermore, the risk of bias in the included studies was not uniformly assessed or reported, raising concerns about the results’ validity. Without a thorough assessment of bias, it is challenging to ascertain the reliability of the findings and their implications for clinical practice.
Lastly, the long-term effects and optimal dosing strategies for Botox in the treatment of spasticity in SCI patients remain inadequately explored. Future research should focus on larger, multicenter trials with standardized outcome measures and rigorous bias assessments to enhance the reliability of findings and guide clinical decision-making.
Ethical Considerations
Compliance with ethical guidelines
This systematic review and meta-analysis was conducted in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments. The information is derived from previously published data.
Funding
This research was supported by a research project funded by Tehran University of Medical Sciences, Tehran, Iran.
Authors contributions
Conceptualization and study design: Seyede Zahra Emami Razavi, Maryam Hosseini, and Mohaddeseh Azadvari; Methodology and data curation: Seyede Zahra Emami Razavi, Maryam Hosseini and Saeed Vaheb; Formal analysis: Saeed Vaheb and Mohsen Rastkar; Writing the original draft: Maryam Hosseini, Mohaddeseh Azadvari, and Mahsa Ghajarzadeh; Review, and editing: Seyede Zahra Emami Razavi, Maryam Cheraghali, and Mahsa Ghajarzadeh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
pinal cord injury (SCI) is a life-altering condition predominantly affecting young individuals and men [1]. This condition not only causes physical impairment but also significantly impacts the psychological, social, and marital status of patients, ultimately interfering with daily activities and diminishing quality of life [2, 3]. Among the most common complications of SCI is spasticity, with a prevalence ranging between 31% and 78% of patients [4, 5]. Spasticity is characterized by intermittent or sustained involuntary muscle activation, which can affect joints such as the shoulders, elbows, wrists, ankles, knees, and hips, leading to abnormal postures and limb pain [5].
Spasticity limits patients’ ability to perform self-care, disrupts sleep, and causes pain, fatigue, and a negative body image, all contributing to a reduced quality of life [4, 5]. The mechanism behind spasticity in SCI arises from disruption in the inhibitory motor pathways, leading to elevated muscle tone and reflexes [4]. In managing spasticity, it is crucial to consider factors like the length of time since injury, the severity of symptoms, patient support systems, and the patient’s and caregivers’ preferences [5, 6]. Oral medications such as baclofen, diazepam, and clonidine are frequently used; however, these therapies often fail to achieve full symptom relief [7].
Botulinum toxin type A (BOTOX®), a neurotoxin produced by Clostridium botulinum, is an approved treatment for spasticity in both upper and lower limbs [5]. This treatment typically benefits 100 to 150 days; patients usually require reinjections to maintain its effects [7].
Previous studies have reported improvement in spasticity following onabotulinumtoxinA injections in SCI patients. However, a systematic review of these studies is needed to synthesize the available evidence and assess the overall efficacy of this treatment.
This study aims to systematically review and evaluate the effects of onabotulinumtoxinA on spasticity in individuals with SCI. The review will also assess the quality of the included studies using predefined criteria. Specifically, the preferred reporting items for systematic reviews and meta-analysis (PRISMA) or the Cochrane risk of bias tool will be employed to evaluate study design, sample size, blinding, and statistical methods. The risk of bias will be reported and discussed in detail to provide transparency regarding the strength of the evidence.
Moreover, although the lack of a meta-analysis is a limitation of this study, the heterogeneity in study designs and outcome measures precluded performing a combined analysis. Future research should aim to standardize outcome measures to facilitate meta-analyses and provide more robust estimates of treatment effects.
Materials and Methods
Search strategy
We conducted a systematic review following the PRISMA guidelines. A comprehensive search of multiple databases, including PubMed, Scopus, EMBASE, Web of Science, and Google Scholar, was performed to identify relevant studies on spasticity treatment in SCI published until September 2023. We also searched gray literature, such as conference abstracts and reference lists of included studies, to ensure a thorough review of all available evidence. The search strategy was designed to cover various studies on pharmacological and non-pharmacological treatments for spasticity, ensuring comprehensive coverage of existing research.
While the Cochrane Library is also a valuable resource, we chose not to include it because it primarily focused on peer-reviewed articles and clinical trials indexed in the broader biomedical databases mentioned. Cochrane reviews primarily aggregate existing systematic reviews, and our objective was to focus on primary research studies that evaluated the efficacy of onabotulinumtoxinA in treating spasticity in SCI patients.
However, the inclusion of Cochrane could be considered in future review updates to ensure an even more comprehensive search strategy.
The MeSH terms that were used for searching in PubMed were as follows:
((“Spinal cord injur*”) OR ((“cord trauma*”) AND (spinal)) OR (“spinal cord trauma*”) OR ((trauma*) AND (“spinal cord”)) OR ((myelopath*) AND (traumatic)) OR (“traumatic myelopath*”) OR ((injur*) AND (spinal cord)) OR ((“cord injur*) AND (spinal)) OR (“spinal cord transection*”) OR ((“cord transection*”) AND (spinal)) OR ((Transection*) AND (“spinal cord”)) OR (“spinal cord laceration*”) OR ((“cord laceration*) AND (spinal)) OR ((laceration*) AND (“spinal cord”)) OR (“post-traumatic myelopath*”) OR ((myelopath*) AND (post-traumatic)) OR (“post traumatic myelopath*”) OR ((contusion*) AND (“spinal cord”)) OR ((“cord contusion*”) AND (spinal)) OR (“spinal cord contusion*”)) AND (((“botulinum toxin*”) AND (“type A”)) OR (“clostridium botulinum A toxin”) OR (“botulinum toxin A”) OR ((“toxin A”) AND (botulinum)) OR (“botulinum neurotoxin A”) OR ((“neurotoxin A”) AND (botulinum)) OR (“botulinum A toxin”) OR ((toxin) AND (“botulinum A”)) OR (“botulinum toxin type A”) OR (“botulinum neurotoxin type A”) OR (“Clostridium botulinum toxin type A”) OR (meditoxin) OR (Botox) OR (neuronox) OR (oculinum) OR (vistabex) OR (onabotulinumtoxina) OR (“onabotulinumtoxin A”) OR (Vistabel) OR (“botulinum toxin*”) OR (“botulinum neurotoxin*”) OR ((toxin*) AND (botulinum)) OR (“clostridium botulinum toxin*”) OR ((toxin*) AND (“Clostridium botulinum”)) OR (“botulinum neurotoxin*”) OR ((neurotoxin*) AND (botulinum)) OR (botulin)).
Inclusion criteria
We included randomized controlled trials (RCTs) or cohort studies that evaluated the efficacy of onabotulinumtoxinA (Botox) in treating spasticity in patients with spinal cord injury.
Exclusion criteria
Studies such as letters to the editor, case-control studies, case reports, and cross-sectional studies were excluded. Cohort studies without a control group were also excluded.
Data extraction
Two independent researchers performed the search, and duplicates were removed before screening the titles and abstracts. In cases of discrepancy, a third researcher was consulted to resolve disagreements. After carefully evaluating the full texts of potentially relevant articles, data were extracted and recorded into data sheets.
Data regarding the total number of participants, first author, publication year, country of origin, mean age, follow-up time, the site of injection, main findings, and side effects were extracted and recorded.
Risk of bias assessment
We assessed the risk of bias in the included studies using two established tools: The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials [8]and the Newcastle-Ottawa scale (NOS) for cohort studies [9].
Cochrane tool: This tool evaluates several specific domains of bias, including:
Selection bias: Assessing how participants were selected and whether the randomization process was adequate.
Performance bias: Examining whether participants and personnel were blinded to the intervention.
Detection bias: Considering if the outcome assessors were blinded to the treatment allocation.
Attrition bias: Evaluating whether there were differences in dropout rates between groups and if the reasons for dropout were reported.
Reporting bias: Analyzing whether the study reported all prespecified outcomes and if any were omitted.
NOS: This scale is used for cohort studies and assesses three domains:
Selection: Evaluating how representative the cohort is and the adequacy of the selection of exposed and non-exposed cohorts.
Comparability: Assessing the comparability of cohorts based on the design or analysis.
Outcome: Considering the assessment of outcomes and whether they were adequately assessed.
The results of the risk of bias assessments will be summarized and discussed in the results section, providing a clear overview of the methodological quality of the included studies and their implications for the findings of this systematic review.
Statistical analysis
No specific statistical analysis was conducted, as this study was designed as a systematic review. However, a meta-analysis could have been performed if the included studies were sufficiently homogeneous regarding study design, interventions, and outcome measures. Unfortunately, conducting a meta-analysis was not feasible due to the significant heterogeneity among the studies, including differences in injection sites, dosing regimens, follow-up periods, and spasticity assessment tools.
It would be beneficial to standardize outcome measures and study designs in future studies to facilitate meta-analyses. Such efforts would allow for more robust quantitative estimates of the effects of onabotulinumtoxinA on spasticity in SCI patients.
Results
A literature search revealed 2914 articles; after deleting duplicates, 1688 remained. After careful evaluation of the full texts, six articles remained (Figure 1). Two studies were from the USA, and the most common site of injection was the upper limbs. The mean age ranged between 36 and 50 years, and the number of patients was between 9 and 112 (Table 1).


In the study by Francisco et al., the patients showed improvement in spasticity and spasticity-related pain after treatment with onabotulinumtoxinA [5]. At the same time, Palazon-Garcia [10] and Spiegl [11] reported improvement in the modified Ashworth scale (MAS) after treatment, which was not in line with the findings of Yan et al. [12]. A decrease in modified Tardieu scale (MTS) and peak knee flexion was reported by Bernuz et al. [13] (Table 1).
Marciniak et al. found improvement in upper-extremity function, positioning, and pain after treatment [14].
Discussion
Spasticity is a disabling complication in patients with SCI. Most published clinical trials regarding the effects of Botox injection on spasticity included patients with stroke or cerebral palsy [15, 16]. This outcome introduces important questions regarding the applicability of the findings to the SCI population. While the pathophysiology of spasticity may share similarities across these conditions, differences in the underlying mechanisms, injury profiles, and patient characteristics can significantly impact treatment responses. Therefore, caution should be exercised when generalizing results from studies focusing on stroke or cerebral palsy patients to those with SCI.
Food and Drug Administration (FDA) approved the administration of Botox for detrusor hyperactivity in patients with SCI in 2011 [13]. As spasticity is generalized in patients with SCI, administration of Botox in muscle groups that dominate the spasticity profile could decrease pain and discomfort related to spasticity [5].
Palazon-Garcia et al. [10] enrolled 90 patients with SCI, while more than 50% received Botox in their lower extremities. Their results showed that Botox injection improved all spasticity-related variables (tone, articular limitations, pain). Specifically, they noted reductions in the MAS scores, indicating decreased muscle tone and improvements in functional mobility assessed through standardized tests. These improvements were noted to last for approximately 12 to 16 weeks following injection. More improvement was observed in patients with incomplete injury and American SCI Association (ASIA) impairment scale D as the administration of Botox was focal in these cases. These findings align with previous research indicating that onabotulinumtoxinA is effective in managing spasticity, particularly in lower extremities, and suggest that this treatment may enhance the overall quality of life for individuals with SCI patients with focal spasticity better improvement [10].
Marciniak et al. enrolled 28 patients with SCI and administered Botox in most flexors of the upper extremities and antigravity muscles of the lower extremities. They reported improvement in ambulation, positioning, function of the upper extremity, and pain. They also found that early administration of Botox vs late administration did not affect the effectiveness [14].
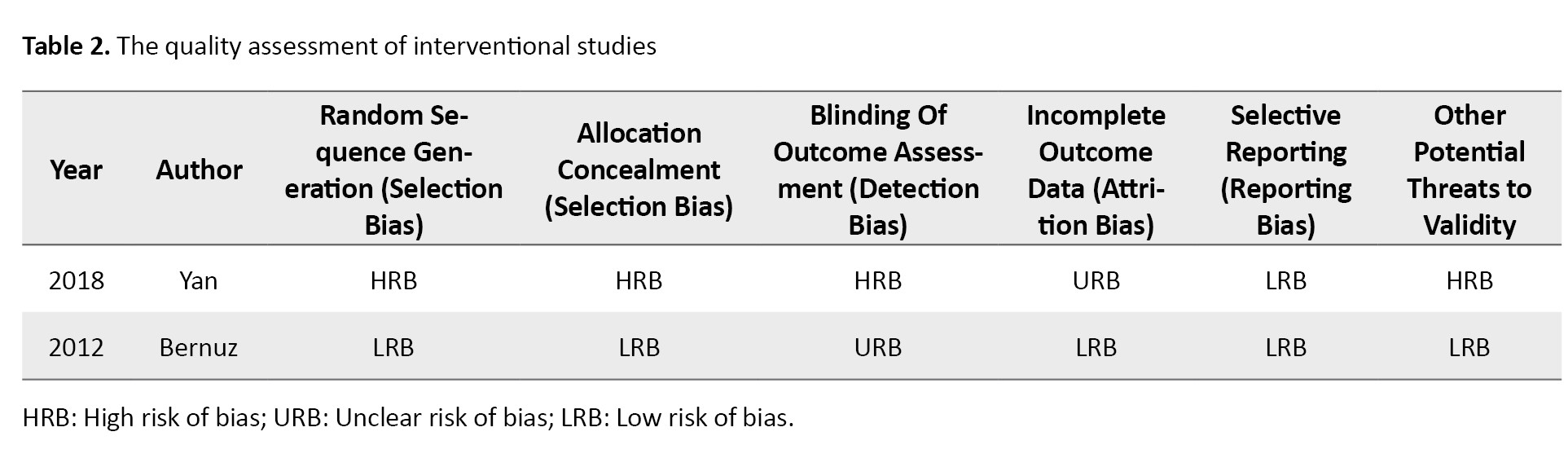
By enrolling 336 patients with SCI in a clinical trial (evaluating baclofen, Botox, and only physical therapy), Yan et al. reported significant improvement in the disability assessment scale (DAS) in the baclofen group compared to the Botox group. This finding highlights the superior efficacy of baclofen in improving functional outcomes, though Botox remains a valuable option for targeted muscle spasticity management [12] (Table 2).

In a study conducted in Germany, Spiegl et al. [11]enrolled 9 patients with traumatic SCI and followed them up for 2 years. The maximum dose of Botox injection administered was 2000 units, and the results were deemed satisfactory in 6 cases. The therapeutic effects of Botox lasted, on average, for approximately 9 months, demonstrating the potential for sustained relief in a subset of patients [11]. However, the small sample size and variability in outcomes suggest a need for further investigation to identify patient-specific factors that may influence the efficacy and duration of Botox treatment.
Spasticity is a common complication following SCI, with 26%-67% of patients developing spasticity during the acute phase. Over time, spasticity becomes even more prevalent, affecting between 46% and 78% of patients after one year [5]. This high incidence makes effective management critical, as spasticity can interfere with daily activities, sleep, social interactions, and overall quality of life. Patients typically seek treatment for spasticity when these symptoms significantly impact their functioning, underscoring the need for timely and effective interventions. Combination therapy, including oral medications, positioning, stretching, blocks, or intrathecal administration of medications, is recommended [6]. Patients with incomplete SCI may have worse experience of spasticity. For patients with focal spasticity, local administration of Botox using injection localization methods such as electromyography (EMG), electrical stimulation (E-stim), and ultrasonography is recommended [7]. The primary effect of Botox is on the neuro-muscular junction, and it also affects the sensory feedback loop [7]. Patients will benefit from decreased pain sensation by improving spasticity and inhibiting the release of P substance [6]. The dosage differs based on the patient’s weight, muscle size, and spasticity degree.
The current management of spasticity involves a combination of therapeutic approaches, including oral medications (such as baclofen or tizanidine), physical positioning and stretching, nerve blocks, or intrathecal administration of medications for more severe cases [6, 7]. It is noteworthy that patients with incomplete SCI may experience more severe and persistent spasticity, making management more challenging.
For focal spasticity, where specific muscle groups are more affected, local administration of botulinum toxin (Botox) is recommended. Injection localization methods, such as EMG, E-stim, and ultrasonography, are critical for ensuring the precise delivery of the toxin to the affected muscles. These techniques improve the accuracy of injections, optimizing the therapeutic effects of Botox and minimizing the risk of complications or suboptimal outcomes [10]. Standardizing these localization methods could enhance treatment efficacy and provide more reliable results for patients with focal spasticity.
One of the rare complications of Botox injection is generalized muscle weakness, which should be considered.
Conclusion
In conclusion, onabotulinumtoxinA appears to be an effective and well-tolerated treatment option for managing spasticity in individuals with spinal cord injury. This systematic review provides compelling evidence for its efficacy in reducing muscle tone and improving function. Given the complexities associated with spasticity in SCI, healthcare providers must adopt a multidisciplinary approach when considering treatment options, incorporating pharmacological, rehabilitative, and supportive strategies tailored to individual patient needs.
Future research should prioritize the standardization of outcome measures to facilitate meta-analyses and the accumulation of more robust evidence regarding the long-term effects of onabotulinumtoxinA in this population. Additionally, larger-scale studies are necessary to explore further this intervention’s efficacy and safety across diverse cohorts of SCI patients, ensuring that treatment modalities are optimized for improving quality of life.
Study limitations
This study has several limitations that should be acknowledged. First, the number of included studies was limited, which restricts the generalizability of the findings to the broader population of patients with SCI. A more extensive range of studies would provide a clearer understanding of the efficacy and safety of onabotulinumtoxinA (Botox) in this context.
Second, the included studies exhibited significant variability in their outcome measures and evaluation methodologies. This inconsistency complicates comparing results across studies and synthesizing data, which may lead to ambiguous conclusions about the treatment’s overall effectiveness.
Moreover, many studies had small sample sizes, which can contribute to variability in findings and may not adequately represent the diverse characteristics of the SCI population. Small sample sizes also limit the power of statistical analyses, potentially obscuring meaningful effects.
Furthermore, the risk of bias in the included studies was not uniformly assessed or reported, raising concerns about the results’ validity. Without a thorough assessment of bias, it is challenging to ascertain the reliability of the findings and their implications for clinical practice.
Lastly, the long-term effects and optimal dosing strategies for Botox in the treatment of spasticity in SCI patients remain inadequately explored. Future research should focus on larger, multicenter trials with standardized outcome measures and rigorous bias assessments to enhance the reliability of findings and guide clinical decision-making.
Ethical Considerations
Compliance with ethical guidelines
This systematic review and meta-analysis was conducted in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments. The information is derived from previously published data.
Funding
This research was supported by a research project funded by Tehran University of Medical Sciences, Tehran, Iran.
Authors contributions
Conceptualization and study design: Seyede Zahra Emami Razavi, Maryam Hosseini, and Mohaddeseh Azadvari; Methodology and data curation: Seyede Zahra Emami Razavi, Maryam Hosseini and Saeed Vaheb; Formal analysis: Saeed Vaheb and Mohsen Rastkar; Writing the original draft: Maryam Hosseini, Mohaddeseh Azadvari, and Mahsa Ghajarzadeh; Review, and editing: Seyede Zahra Emami Razavi, Maryam Cheraghali, and Mahsa Ghajarzadeh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Ghajarzadeh M, Saberi H. Transportation mode and timing of spinal cord decompression and stabilization in patients with traumatic spinal cord injury in Iran. Spinal Cord. 2019; 57(2):150-5. [DOI:10.1038/s41393-018-0189-5] [PMID]
- Saberi H, Ghajarzadeh M. Emotional Intelligence in Patients with Spinal Cord Injury (SCI). Iranian Journal of Public Health. 2017; 46(5):677-81. [PMID]
- Ghajarzadeh M, Saberi H. Association between depression and chronic complications in clients with traumatic spinal cord injury. Acta Medica Iranica. 2019; 56(11):704-9. [Link]
- Angulo-Parker FJ, Adkinson JM. Common etiologies of upper extremity spasticity. Hand Clinics. 2018; 34(4):437-43. [DOI:10.1016/j.hcl.2018.06.001] [PMID]
- Francisco GE, Bandari DS, Bavikatte G, Jost WH, McCusker E, Largent J, et al. High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study. Toxicon X. 2020; 7:100040. [DOI:10.1016/j.toxcx.2020.100040] [PMID]
- Agarwal S, Patel T, Shah N, Patel BM. Comparative study of therapeutic response to baclofen vs tolperisone in spasticity. Biomedicine & Pharmacotherapy. 2017; 87:628-35. [DOI:10.1016/j.biopha.2017.01.005] [PMID]
- Dai AI, Aksoy SN, Demiryürek AT. Comparison of efficacy and side effects of oral baclofen versus tizanidine therapy with adjuvant botulinum toxin type A in children with cerebral palsy and spastic equinus foot deformity. Journal of Child Neurology. 2016; 31(2):184-9. [DOI:10.1177/0883073815587030] [PMID]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. [DOI:10.1136/bmj.d5928] [PMID]
- Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. Newcastle-Ottawa quality assessment scale cohort studies. Ottawa: University of Ottawa; 2014. [Link]
- Palazón-García R, Alcobendas-Maestro M, Esclarin-de Ruz A, Benavente-Valdepeñas AM. Treatment of spasticity in spinal cord injury with botulinum toxin. The Journal of Spinal Cord Medicine. 2019; 42(3):281-7. [DOI:10.1080/10790268.2018.1479053] [PMID]
- Spiegl UJ, Maier D, Gonschorek O, Heyde CE, Bühren V. Antispastic therapy with botulinum toxin type A in patients with traumatic spinal cord lesion. GMS Interdisciplinary Plastic and Reconstructive Surgery DGPW. 2014; 3:Doc14. [PMID]
- Yan X, Lan J, Liu Y, Miao J. Efficacy and safety of botulinum toxin type A in spasticity caused by spinal cord injury: A randomized, controlled trial. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2018; 24:8160-71. [DOI:10.12659/MSM.911296] [PMID]
- Bernuz B, Genet F, Terrat P, Pradon D, Barbot F, Bussel B, et al. Botulinum toxin effect on voluntary and stretch reflex-related torque produced by the quadriceps: an isokinetic pilot study. Neurorehabil Neural Repair. 2012; 26(5):542-7. [PMID]
- Marciniak C, Rader L, Gagnon C. The use of botulinum toxin for spasticity after spinal cord injury. American Journal of Physical Medicine & Rehabilitation. 2008; 87(4):312-7; quiz 318-20, 329. [DOI:10.1097/PHM.0b013e318168ceaf] [PMID]
- Kaji R, Osako Y, Suyama K, Maeda T, Uechi Y, Iwasaki M, et al. Botulinum toxin type A in post-stroke lower limb spasticity: A multicenter, double-blind, placebo-controlled trial. Journal of Neurology. 2010; 257(8):1330-7. [DOI:10.1007/s00415-010-5526-3] [PMID]
- Garreta-Figuera R, Chaler-Vilaseca J, Torrequebrada-Giménez A. [Clinical practice guidelines for the treatment of spasticity with botulinum toxin (Spanish)]. Revista de Neurologia. 2010; 50(11):685-99. [DOI:10.33588/rn.5011.2010174] [PMID]
Type of Study: Review Article |
Subject:
Neurology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |