Volume 13, Issue 1 (Winter 2025)
Iran J Health Sci 2025, 13(1): 1-10 |
Back to browse issues page
Ethics code: N/A
Clinical trials code: N/A
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yugi Owiti J, Khatoro Taabu R, Odhiambo Sylvester R, Gitonga Mawira N, Owuor Onyango B. Characterization and Larvicidal Potency of Crude Urtica massaica Extracts Against Anopheles gambiae (Diptera: Culicidae). Iran J Health Sci 2025; 13 (1) :1-10
URL: http://jhs.mazums.ac.ir/article-1-973-en.html
URL: http://jhs.mazums.ac.ir/article-1-973-en.html
Jared Yugi Owiti * 

 , Rose Khatoro Taabu
, Rose Khatoro Taabu 

 , Rechab Odhiambo Sylvester
, Rechab Odhiambo Sylvester 

 , Nicholas Gitonga Mawira
, Nicholas Gitonga Mawira 

 , Bethwell Owuor Onyango
, Bethwell Owuor Onyango 




 , Rose Khatoro Taabu
, Rose Khatoro Taabu 

 , Rechab Odhiambo Sylvester
, Rechab Odhiambo Sylvester 

 , Nicholas Gitonga Mawira
, Nicholas Gitonga Mawira 

 , Bethwell Owuor Onyango
, Bethwell Owuor Onyango 


Department of Biological Sciences, School of Science and Technology, University of Kabianga, Kericho, Kenya. , jowiti@kabianga.ac.ke
Full-Text [PDF 1007 kb]
(270 Downloads)
| Abstract (HTML) (583 Views)
Full-Text: (131 Views)
Introduction
Plant secondary metabolites or bioactive compounds are products secreted by plants for defense against plant natural enemies. The products serve as repellents, antifeedants, growth and mating inhibitors, anti-moulting, and insecticides [1, 2] against these enemies. The products, however, are environmentally friendly [3] and serve as food and preservatives [4]. The bioactive compounds are diverse with different modes of action. This property gives them great potential for novel biological products [5] and makes it challenging for diseases and pests to develop resistance against them [6]. The tragedy is that most products remain unexplored and serve merely as repository sources [7].
Urtica massaica Mildbr (hereafter U. massaica) is a perennial herb [8] belonging to the Urticaceae family [9]and, although considered a weed, is among the most used wild plant species in the world [10, 11]. It is rich in proteins [12], vitamins [13], and polyphenols [14], and it has pharmaceutical [15], antimicrobial [16], and fungicidal [17] potential. It has, therefore, been used as vegetables with high potential to manage food insecurity [18-19] and as an herb to treat various microorganism-caused infections [20-23], stomach ulcers, hypertension, nerve disorders, diabetes, and rheumatism [4, 24].
Though the toxic effect of the extracts has been reported on immature Anopheles gambiae (hereafter A. gambiae) [25], the chemical profile and toxicity have not been studied. We, therefore, report herein on the characterization and potency of crude methanol and hexane extracts of U. massaica against laboratory-cultured A. gambiae larvae under controlled conditions.
Materials and Methods
Study site, sourcing for experimental mosquitoes and study design
This research was a laboratory-based experimental bioassay using crude U. massaica extracts on A. gambiae larvae. We extracted and characterized chemical constituents from parts of U. massaica using methanol and hexane in the University of Eldoret’s chemistry laboratories. Third larval instars (L3s) of A. gambiae mosquitoes were obtained from a laboratory stock at the Centre for Global Health Research/Kenya Medical Research Institute (CGHR/KEMRI), Kisian, Kisumu. Since the effect of treatment meted on the larvae was measured only after the bioassay, an informal ‘after-only with control’ experimental design [26] was used to determine the larvicidal effect of crude methanol and hexane extracts of U. massaica on the larvae.
Plant materials
Fresh leaves, stems, and roots of U. massaica (stinging nettle) were sourced from 350 16’ 46” E, 00 31’ 41” N in Eldoret. The plant was identified, and a voucher specimen number JOY2021/001 was issued and later deposited at the School of Biological Sciences, University of Nairobi, herbarium.
Methanol and hexane extracts of U. massaica
Two hundred grams of ground leaves of U. massaica were soaked in 400 mL of absolute methanol for 1 hour. The suspension was then filtered using Whitman’s No. 1 filter paper, and the filtrates were freeze-dried using the Edwards Modulyo freeze-drying machine. The result is a paste taken as stock material [27]. This procedure was repeated for ground stem and roots and hexane solvent.
Gas chromatography-mass spectrometry (GC-MS) analysis
Sample preparation
This procedure was done as described elsewhere [28]. Briefly, 1 mg of each crude extract was weighed (in triplicates) and dissolved in 1 mL dichloromethane. The samples were vortexed for 10 s, ultra-sonicated for 1 hour, and centrifuged at 14000 rpm. The supernatant was dried using anhydrous Na2SO4 and centrifuged at 14000 rpm before GC-MS analysis.
Instrument conditions
This activity was conducted as described elsewhere [29]. Briefly, the samples were analyzed using a 7890A gas chromatograph connected to a 5975C mass selective detector. A temperature of 270 °C was set as the inlet and 280 °C as the transfer line. The oven temperature was programmed at between 35 °C and 285 °C, with the initial temperature being maintained for 5 minutes, adjusted to 10 °C/minute, and progressively brought up to and held at 280 °C for half an hour. A low bleed capillary column (HP-5 MS, 30 m × 0.25 mm i.d., 0.25 µm) and helium were employed as the carrier gas with a 1.25 mL/min flow rate. The detector’s ion source and quadrupole temperatures were maintained at 230 °C and 180 °C, respectively. Electron impact of mass spectra was obtained at 70 eV. About 1.0 µL and the analyte injected in split/splitless mode and fragmented ions analyzed, in full scan mode, over 40 to 550 m/z range. The filament delay time for the analytes was set at 3.3 min. The compounds were identified by comparing their fragmentation patterns with reference spectra from Library–MS databases. This process also included the National Institute of Standards and Technology (NIST) 11, 08, and 05, Adams and Chemical mass spectral databases.
Liquid chromatography with tandem mass spectrometry analysis
The analysis was conducted as described elsewhere [30]. Three replicates of 1 mg of each crude methanolic extract were weighed and constituted in 1 mL methanol. The samples were then vortexed for 10 s ultrasonicated for 1 h before centrifugation at 14000 rpm. Thereafter, the supernatant was filtered and analyzed qualitative tandem liquid chromatography quadrupole time of flight mass spectrometry (MeOH) under the following conditions: Ultra-performance liquid chromatography (UPLC) (Waters ACQUITY I-Class system); UPLC column (Waters ACQUITY UPLC BEH C18 column, 2.1×50 mm, 1.7-μm particle size Waters Corporation, Dublin, Ireland); Column temperature of 25 °C; mobile phase of water (A) and methanol (B), each with 0.01% formic acid; flow rate of 0.3 mL/min, gradient from 95% A to 100% B and back to starting solvent proportion; with the run time being 25 min. A positive Q-tof ion mode with a nitrogen desolvation flow rate of 500 L/h and an accuracy of <5 ppm was used. The quantitative analysis of the secondary metabolites present was based on a standard curve of apigenin (y=10288x–11117; R2=0.999).
Larvicidal bioassay
Preparation of serial dilutions
The dilutions were prepared according to Vloemans et al. [31]. Briefly, 1 g of crude methanol stock’s extracts of U. massaica leaves were weighed and dissolved in 1% of dimethyl sulfoxide (100 mL) and then serially diluted. Also, 80 mL of this solution was then topped up with 20 mL of distilled water to make 100 mL. The mixture was then serially diluted to 40, 20, 10, 5, and 2.5 mL/100 cm3 (e/w) of distilled water.
Larviciding
One hundred newly transformed L3s were placed in 3 plastic containers, each measuring 6×5.7×3.5 cm, using a plastic pipette. Two sets of the containers held 33, while the third had 34 larvae each. All the containers contained similar doses of a particular treatment. Each container held approximately 33 mL of a particular dose. Doses were either 80, 40, 20, 10, 5, or 2.5 mL/100 mL (e/w). The larvae were left exposed overnight, after which the experiment was stopped, and the number of dead or live individuals was noted and recorded. All larvae (live, moribund, and dead) were then put in a pail of hot water and dispensed in a septic tank. The experiment was replicated four times. Larvicidal activities were tested following the World Health Organization (WHO) procedure [32] and standards [33] for insecticidal effectiveness. Larval mortality was calculated using the Equation 1:
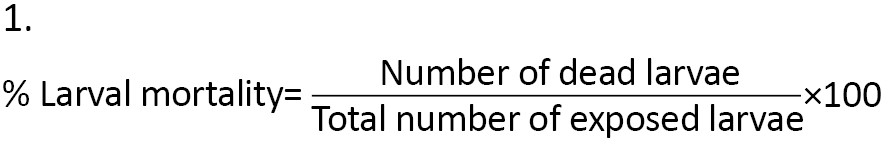
Furthermore, observed larval mortality of between 5% and 20% was corrected using Abbot’s formula [34] (Equation 2):
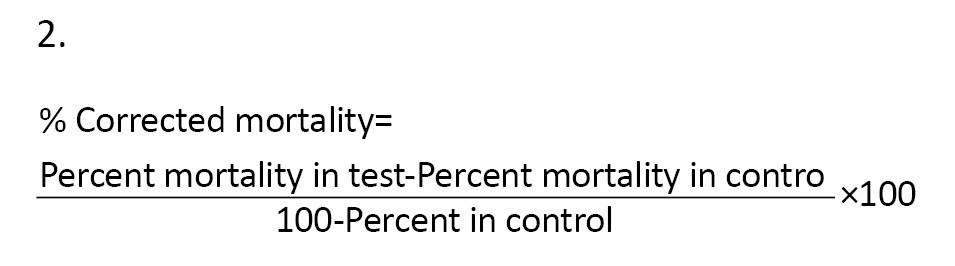
The extract dose was taken as the independent variable, while observed mortalities were the dependent variable. Dimethyl sulfoxide and distilled water were used as negative controls. The temperature and humidity regime at the laboratory was maintained at 28 – 30 °C and 70% - 80%, respectively, and a photoperiod at 12 h light (06.30 – 18.30 hours) alternated with 12 h darkness (18:30 – 06:30 hours).
Statistical analysis
Data on characterization and bioassay on the effect of the crude extracts of U. massaica on larvae of A. gambiae were entered in Excel spreadsheets and appropriately organized for processing. Descriptive statistics were used to determine the quantity of bioactive compounds and the effect of the solvent of extraction, dose, and part of the plant used on exposed larvae. A one-way analysis of variance was used to determine the significance of the impact of extract as a larvicide. All statistical analysis was performed using IBM SPSS statistics software for Windows, version 22 (IBM Corp, Armonk, NY, 2013).
Results
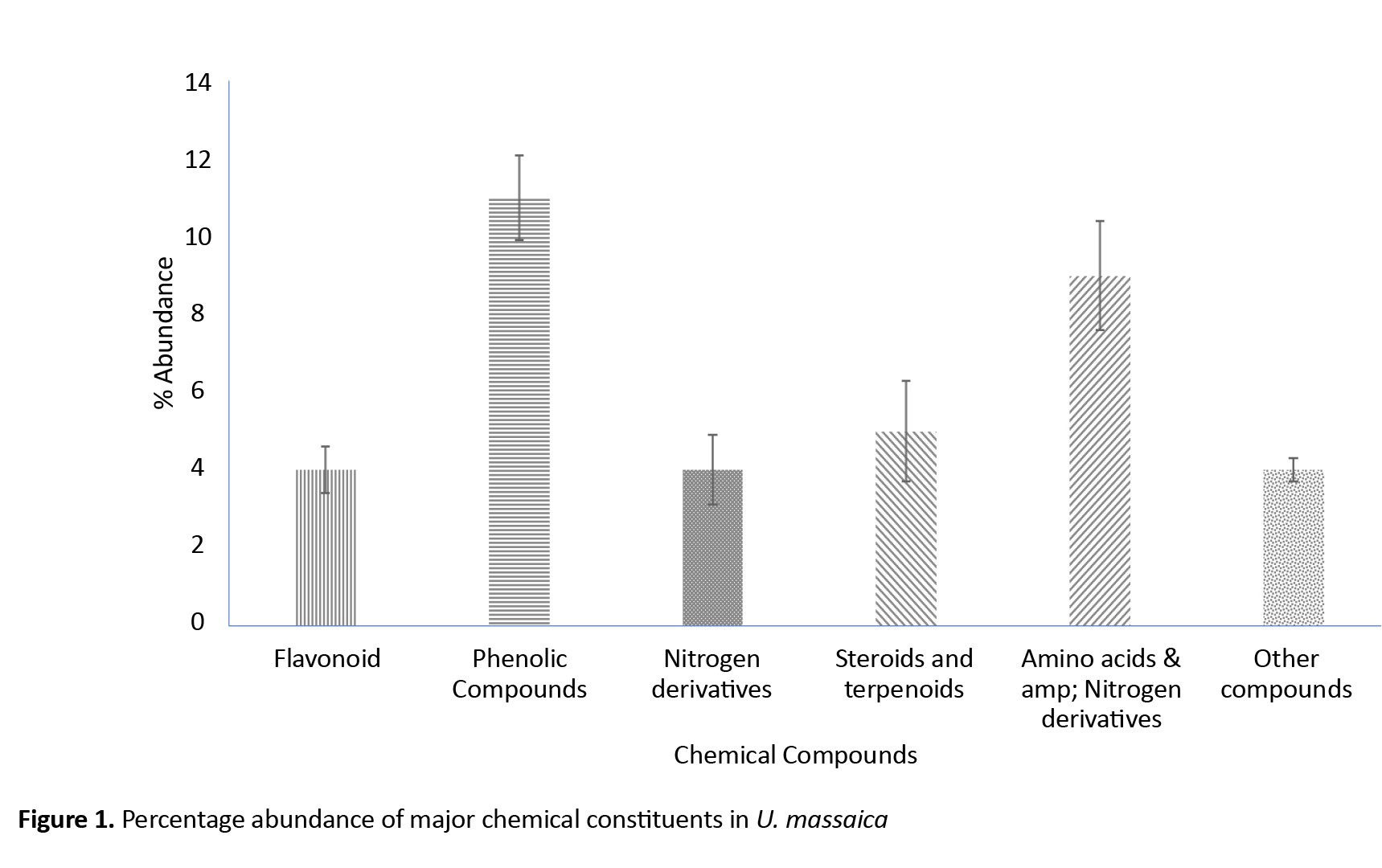
Five major chemical constituents were extracted from U. massaica parts. Those that could not be classified were placed under “other groups.” The order of abundance was phenolic > amino acids and nitrogen derivatives > steroids and terpenoids > flavonoids, nitrogen derivatives, and other groups (Figure 1). Roots produced the highest quantity of constituents [12], followed by leaves [16] and then stems [12]. All the observed abundance, however, did not significantly differ (P<0.05) except for the flavonoids (Table 1).
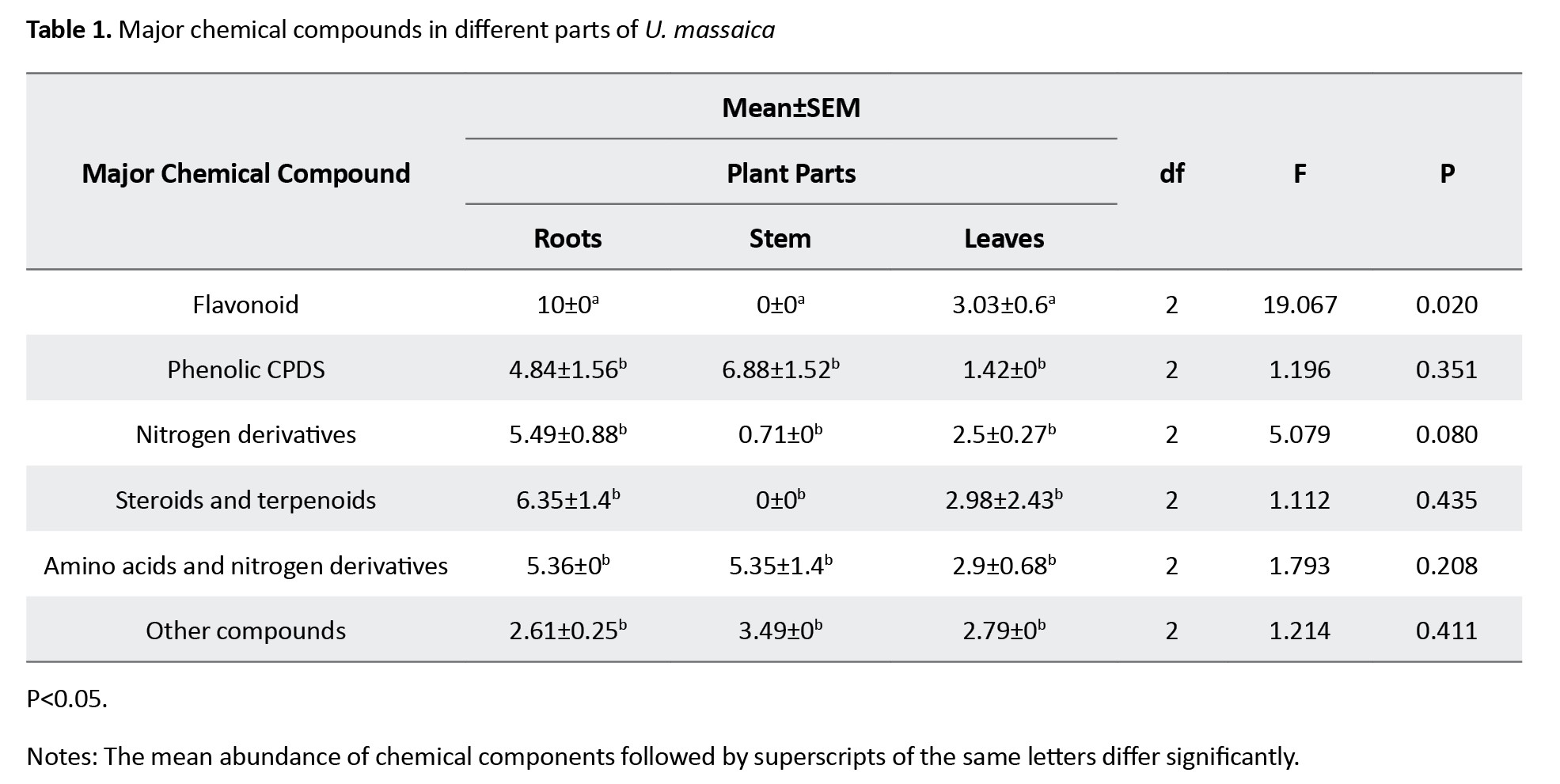
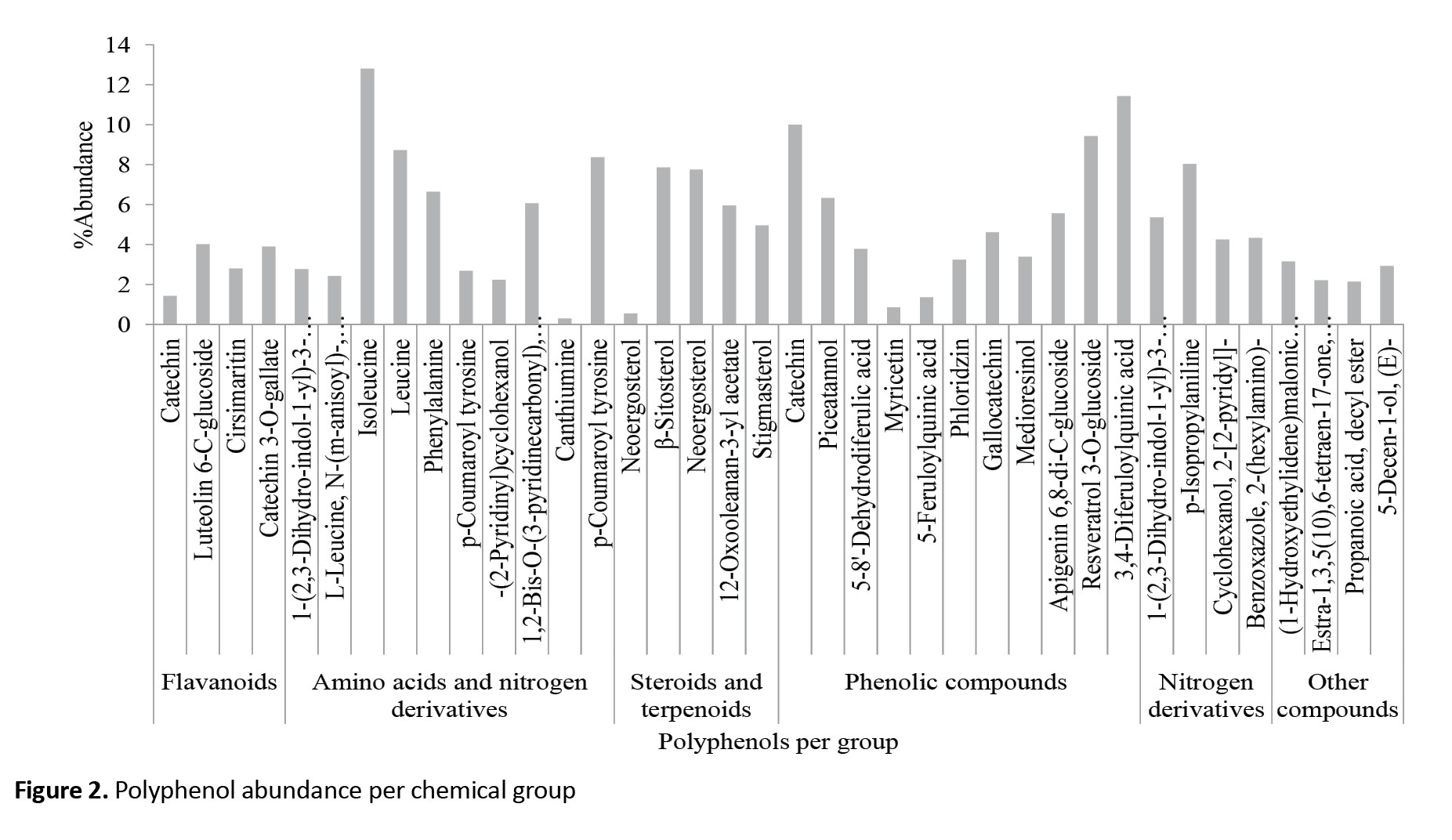
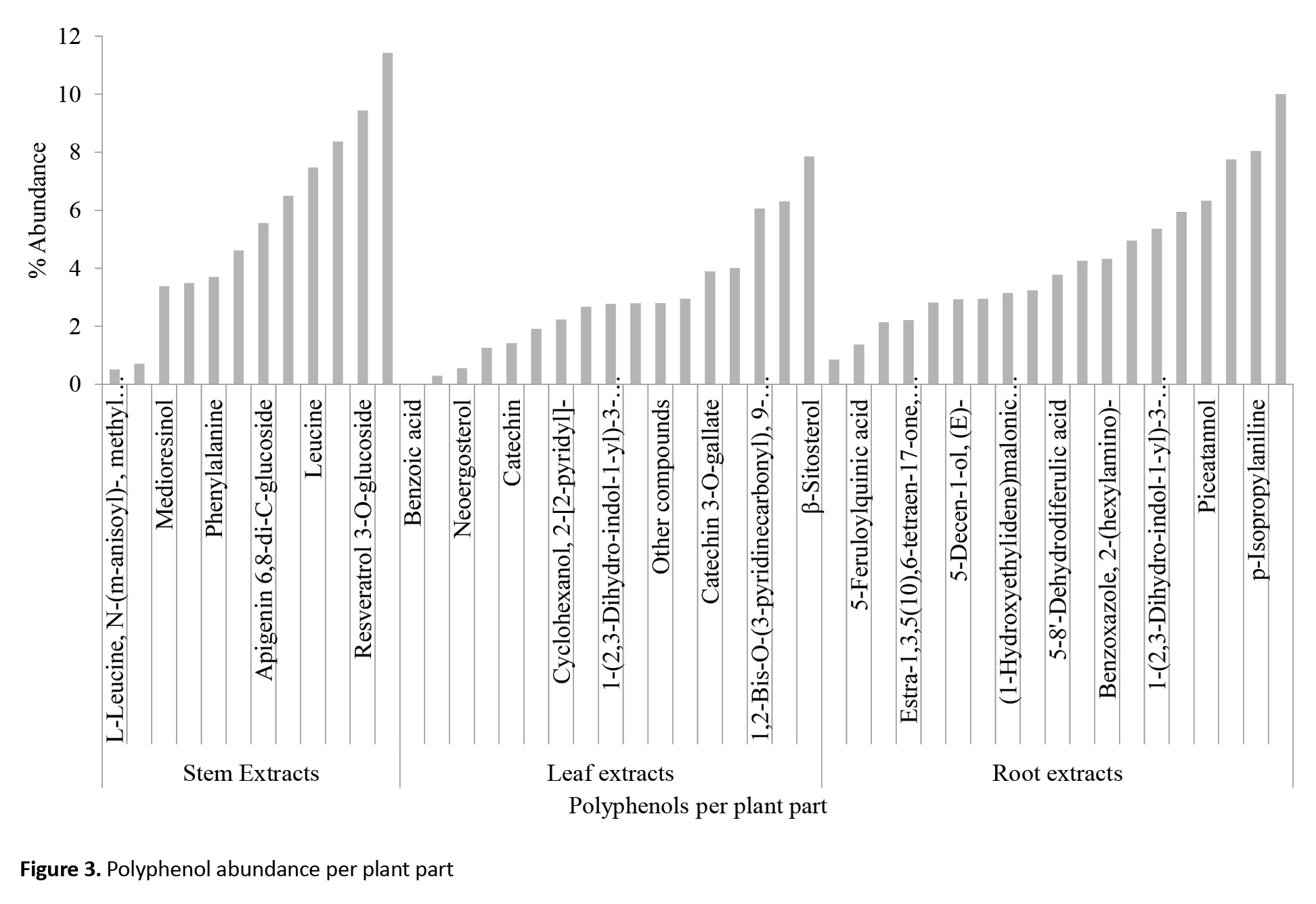
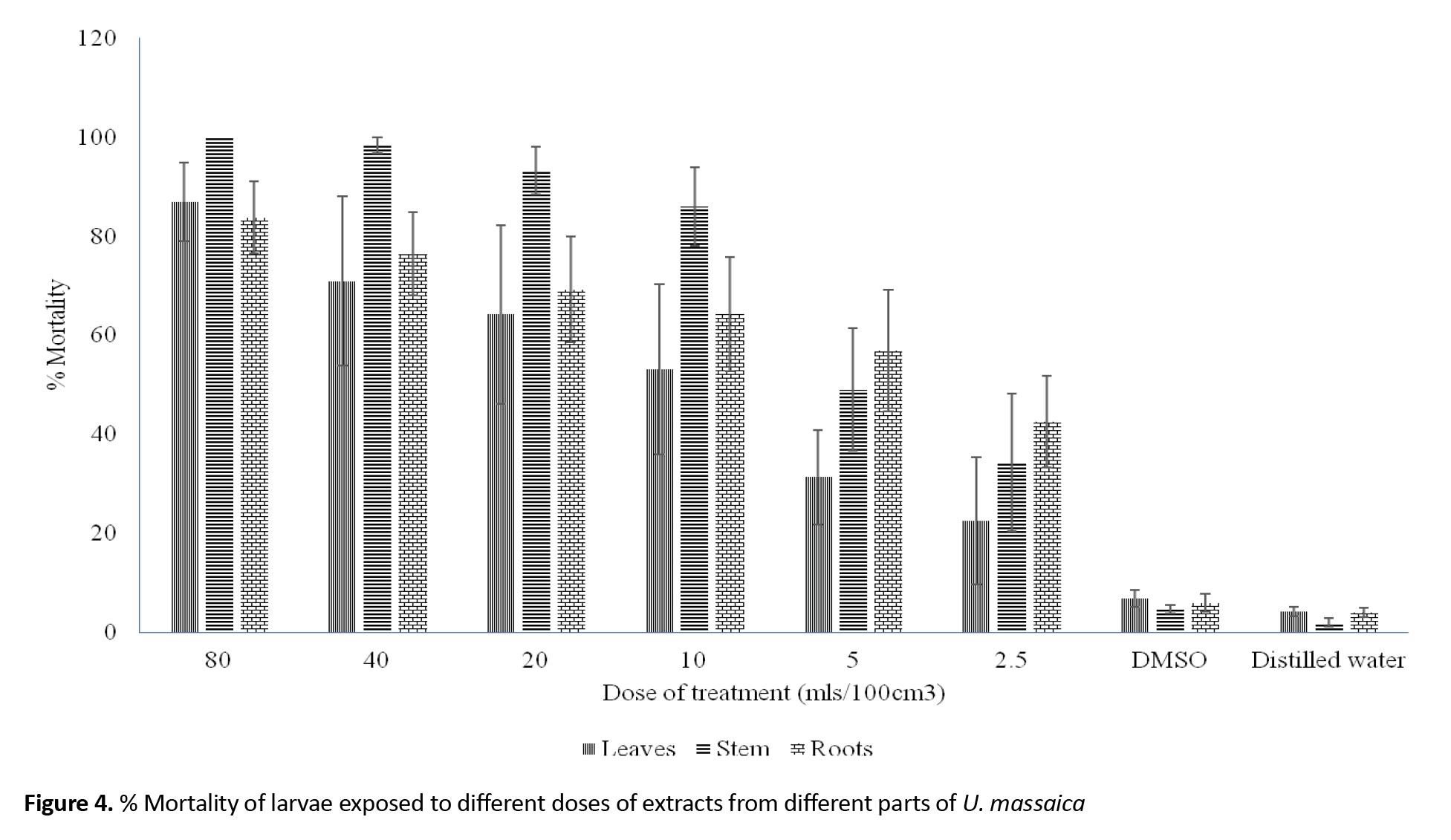
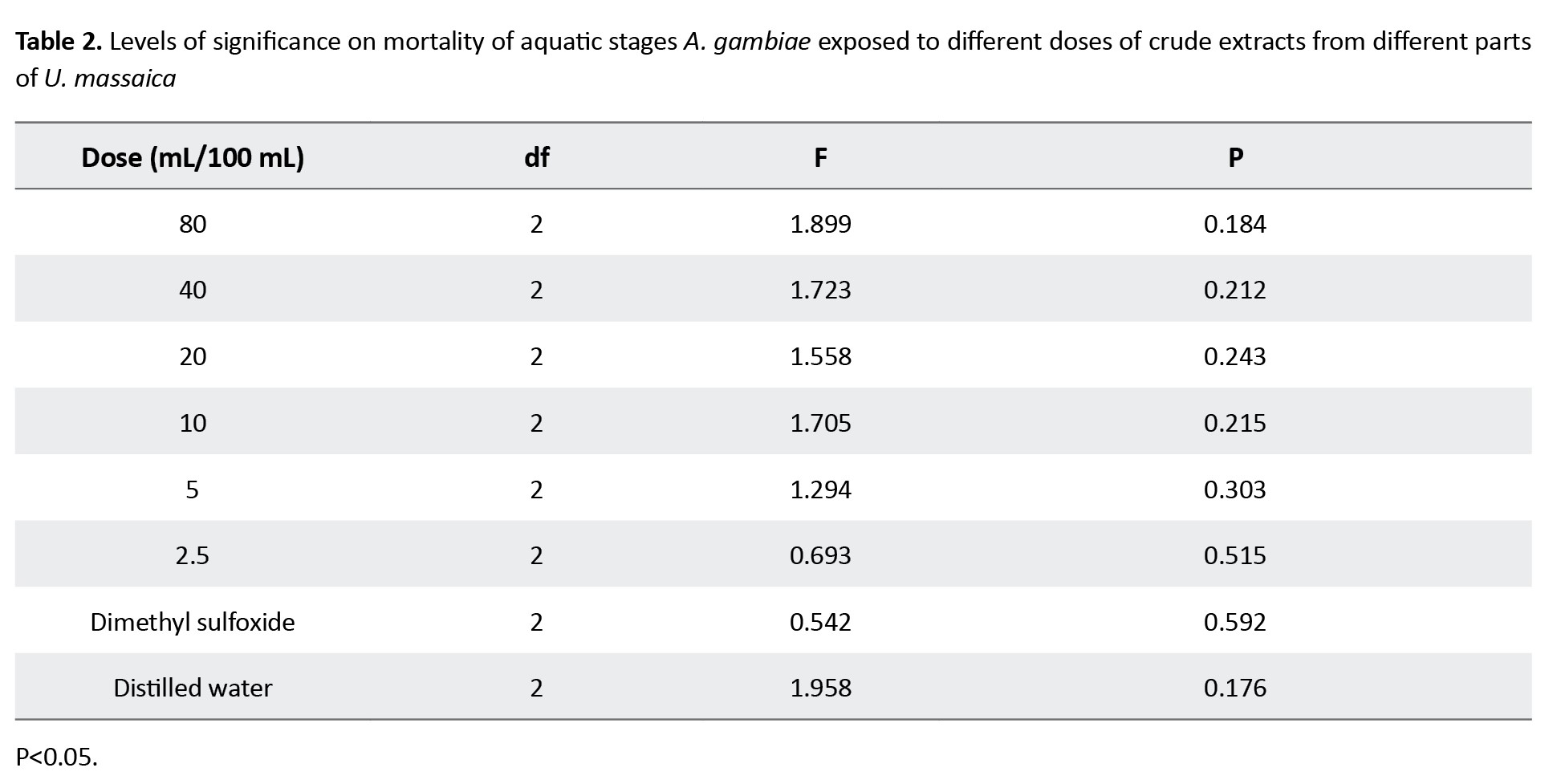
Individual chemical constituents, however, differed in abundance per chemical group and plant part. Luteolin 6-C-glucoside (flavonoids), neoergosterol and β-sitosterol (steroids and terpenoids), isoleucine (amino acid and nitrogen derivatives), 3,4-diferuloylquinic acid (phenolic compounds) and p-isopropyl aniline (nitrogen derivatives) (Figure 2) were abundant per group and catechin (10.00) in roots, β-sitosterol (7.84) in leaf and 3,4-diferuloylquinic acid (11.43) in stem (Figure 3). Interestingly, extracts from stems were not only more potent than those from leaves or roots, but their effects equaled the WHO threshold of >80% mortality for insecticidal effectiveness for doses of 10 mL/100 cm3 (e/w) and above (Figure 4). Observed mortalities, however, were not significantly different (P>0.05) irrespective of dose or control (Table 2).
Discussion
Different parts of plants (leaves, fruits, seeds, roots, and bark) contain polyphenols or secondary metabolites (flavonols, anthocyanins, and phenolic acids) that are responsible for mosquitocidal properties [35]. Indeed, much of the research on malaria intervention is on the search for novel chemical agents that have equivalent potency as synthetic insecticides but whose activity is specific and friendly to humans and the environment [35, 36]. The study reported herein was one of such, and herein we demonstrate that U. massaica contains several chemical agents that are toxic to A. gambiae larvae. The findings are similar to Justicia adhatoda L. [37] and Acacia nilotica [38] against different mosquito species.
In this study, the roots contained more bioactive compounds than the leaves or stem, though extracts from the stem were more potent than those of the leaves or roots. These findings were similar to those of Baz et al. [39], Anupam et al. [40], and Yugi and Kiplimo [41], who reported on the differential distribution and concentration of bioactive compounds in different plant parts. These findings come when insecticide resistance and environmental challenges [42] posed by synthetic insecticides are a thorn in the flesh against the race to reduce, and if not pacified, malaria infection must be welcomed. Indeed, it adds to the cumulated stock and use of natural products that are not only rich in bioactive compounds but are target-specific and safe for the environment [3, 36]. As such, the findings of this study put extracts of U. massaica among plants that can provide alternative sources of green insecticides [43] for mosquito-borne disease control [33].
The study showed that U. massaica contains secondary metabolites identified as tannins, terpenes, saponins, flavonoids, alkaloids, and phenols. A total of 47 bioactive compounds were extracted in different quantities (roots, 19; leaves, 16; and stem, 12) of the U. massaica plant. This amount was moderate but still higher than recently reported elsewhere [14]. This finding is promising as we believe that with refining, we are likely to realize more compounds. Though the exact nature and the specific bioactive compound responsible for the observed activity were not expressly determined, it is evident from an earlier demonstration that phenolic compounds, flavonoids, and tannins possess insecticidal properties [44] and may have been responsible for the observed toxicity. These findings are comparable with earlier works by Hoesain et al. [45] of plant metabolite potential against Spodoptera litura, Hillary et al. [46] of efficacy of plant products, Bassey et al. [47] of Allium sativum and Murraya koenigii, and Folawewo et al. [48], Oboho et al. [49] of Hippocratea africana with demonstrable mosquitocidal [50] activities. The bioactive compounds’ toxicity was believed to be due to their impairing mitochondrial function in the exposed insect vectors [51].
The present study demonstrates the toxicity of extracts of U. massaica against A. gambiae larvae with activity being dependent on dose, solvent, and part of plant extracted. Though the time of exposure was not tested, the observations reported here agree with those reported by Ubulom et al. [51], Opara et al. [52], and Ghosh et al. [40] on the count of increasing concentration of bioactive compounds in different parts of the plant.
Conclusion
U. massaica bioactive compounds are diverse and have toxicants against A. gambiae larvae. They qualify for exploitation as green insecticides to target and protect against the anthropophilic, endophilic, and endophagic mosquitoes in areas where they are endemic and resist synthetic insecticides.
Study limitations
The study was limited to L3 of laboratory-reared A. gambiae mosquitoes.
Ethical Considerations
Compliance with ethical guidelines
This research was a laboratory-based experimental bioassay. It involved the use of laboratory-sourced and membrane-fed mosquitoes only. There was, therefore, no need for ethical consent.
Funding
The University of Kabianga, Kericho, Kenya funded the project.
Authors contributions
Conceptualization, data analysis, and writing the original draft: Jared Yugi Owiti, supervision, methodology, investigation, data collection, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Richard Amito, Harnel Versey, and Gayle Aurelia for processing and culturing the experimental mosquitoes. Also, the authors appreciate the Centre for Global Health Research (CGHR), Kisumu, for providing laboratory space, experimental mosquitoes, and equipment, respectively.
References
Plant secondary metabolites or bioactive compounds are products secreted by plants for defense against plant natural enemies. The products serve as repellents, antifeedants, growth and mating inhibitors, anti-moulting, and insecticides [1, 2] against these enemies. The products, however, are environmentally friendly [3] and serve as food and preservatives [4]. The bioactive compounds are diverse with different modes of action. This property gives them great potential for novel biological products [5] and makes it challenging for diseases and pests to develop resistance against them [6]. The tragedy is that most products remain unexplored and serve merely as repository sources [7].
Urtica massaica Mildbr (hereafter U. massaica) is a perennial herb [8] belonging to the Urticaceae family [9]and, although considered a weed, is among the most used wild plant species in the world [10, 11]. It is rich in proteins [12], vitamins [13], and polyphenols [14], and it has pharmaceutical [15], antimicrobial [16], and fungicidal [17] potential. It has, therefore, been used as vegetables with high potential to manage food insecurity [18-19] and as an herb to treat various microorganism-caused infections [20-23], stomach ulcers, hypertension, nerve disorders, diabetes, and rheumatism [4, 24].
Though the toxic effect of the extracts has been reported on immature Anopheles gambiae (hereafter A. gambiae) [25], the chemical profile and toxicity have not been studied. We, therefore, report herein on the characterization and potency of crude methanol and hexane extracts of U. massaica against laboratory-cultured A. gambiae larvae under controlled conditions.
Materials and Methods
Study site, sourcing for experimental mosquitoes and study design
This research was a laboratory-based experimental bioassay using crude U. massaica extracts on A. gambiae larvae. We extracted and characterized chemical constituents from parts of U. massaica using methanol and hexane in the University of Eldoret’s chemistry laboratories. Third larval instars (L3s) of A. gambiae mosquitoes were obtained from a laboratory stock at the Centre for Global Health Research/Kenya Medical Research Institute (CGHR/KEMRI), Kisian, Kisumu. Since the effect of treatment meted on the larvae was measured only after the bioassay, an informal ‘after-only with control’ experimental design [26] was used to determine the larvicidal effect of crude methanol and hexane extracts of U. massaica on the larvae.
Plant materials
Fresh leaves, stems, and roots of U. massaica (stinging nettle) were sourced from 350 16’ 46” E, 00 31’ 41” N in Eldoret. The plant was identified, and a voucher specimen number JOY2021/001 was issued and later deposited at the School of Biological Sciences, University of Nairobi, herbarium.
Methanol and hexane extracts of U. massaica
Two hundred grams of ground leaves of U. massaica were soaked in 400 mL of absolute methanol for 1 hour. The suspension was then filtered using Whitman’s No. 1 filter paper, and the filtrates were freeze-dried using the Edwards Modulyo freeze-drying machine. The result is a paste taken as stock material [27]. This procedure was repeated for ground stem and roots and hexane solvent.
Gas chromatography-mass spectrometry (GC-MS) analysis
Sample preparation
This procedure was done as described elsewhere [28]. Briefly, 1 mg of each crude extract was weighed (in triplicates) and dissolved in 1 mL dichloromethane. The samples were vortexed for 10 s, ultra-sonicated for 1 hour, and centrifuged at 14000 rpm. The supernatant was dried using anhydrous Na2SO4 and centrifuged at 14000 rpm before GC-MS analysis.
Instrument conditions
This activity was conducted as described elsewhere [29]. Briefly, the samples were analyzed using a 7890A gas chromatograph connected to a 5975C mass selective detector. A temperature of 270 °C was set as the inlet and 280 °C as the transfer line. The oven temperature was programmed at between 35 °C and 285 °C, with the initial temperature being maintained for 5 minutes, adjusted to 10 °C/minute, and progressively brought up to and held at 280 °C for half an hour. A low bleed capillary column (HP-5 MS, 30 m × 0.25 mm i.d., 0.25 µm) and helium were employed as the carrier gas with a 1.25 mL/min flow rate. The detector’s ion source and quadrupole temperatures were maintained at 230 °C and 180 °C, respectively. Electron impact of mass spectra was obtained at 70 eV. About 1.0 µL and the analyte injected in split/splitless mode and fragmented ions analyzed, in full scan mode, over 40 to 550 m/z range. The filament delay time for the analytes was set at 3.3 min. The compounds were identified by comparing their fragmentation patterns with reference spectra from Library–MS databases. This process also included the National Institute of Standards and Technology (NIST) 11, 08, and 05, Adams and Chemical mass spectral databases.
Liquid chromatography with tandem mass spectrometry analysis
The analysis was conducted as described elsewhere [30]. Three replicates of 1 mg of each crude methanolic extract were weighed and constituted in 1 mL methanol. The samples were then vortexed for 10 s ultrasonicated for 1 h before centrifugation at 14000 rpm. Thereafter, the supernatant was filtered and analyzed qualitative tandem liquid chromatography quadrupole time of flight mass spectrometry (MeOH) under the following conditions: Ultra-performance liquid chromatography (UPLC) (Waters ACQUITY I-Class system); UPLC column (Waters ACQUITY UPLC BEH C18 column, 2.1×50 mm, 1.7-μm particle size Waters Corporation, Dublin, Ireland); Column temperature of 25 °C; mobile phase of water (A) and methanol (B), each with 0.01% formic acid; flow rate of 0.3 mL/min, gradient from 95% A to 100% B and back to starting solvent proportion; with the run time being 25 min. A positive Q-tof ion mode with a nitrogen desolvation flow rate of 500 L/h and an accuracy of <5 ppm was used. The quantitative analysis of the secondary metabolites present was based on a standard curve of apigenin (y=10288x–11117; R2=0.999).
Larvicidal bioassay
Preparation of serial dilutions
The dilutions were prepared according to Vloemans et al. [31]. Briefly, 1 g of crude methanol stock’s extracts of U. massaica leaves were weighed and dissolved in 1% of dimethyl sulfoxide (100 mL) and then serially diluted. Also, 80 mL of this solution was then topped up with 20 mL of distilled water to make 100 mL. The mixture was then serially diluted to 40, 20, 10, 5, and 2.5 mL/100 cm3 (e/w) of distilled water.
Larviciding
One hundred newly transformed L3s were placed in 3 plastic containers, each measuring 6×5.7×3.5 cm, using a plastic pipette. Two sets of the containers held 33, while the third had 34 larvae each. All the containers contained similar doses of a particular treatment. Each container held approximately 33 mL of a particular dose. Doses were either 80, 40, 20, 10, 5, or 2.5 mL/100 mL (e/w). The larvae were left exposed overnight, after which the experiment was stopped, and the number of dead or live individuals was noted and recorded. All larvae (live, moribund, and dead) were then put in a pail of hot water and dispensed in a septic tank. The experiment was replicated four times. Larvicidal activities were tested following the World Health Organization (WHO) procedure [32] and standards [33] for insecticidal effectiveness. Larval mortality was calculated using the Equation 1:
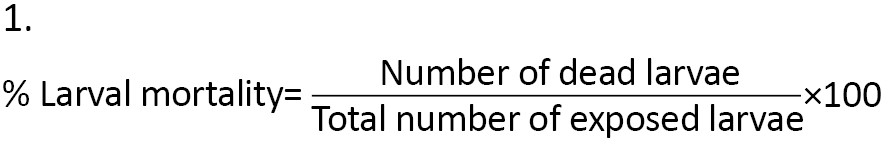
Furthermore, observed larval mortality of between 5% and 20% was corrected using Abbot’s formula [34] (Equation 2):
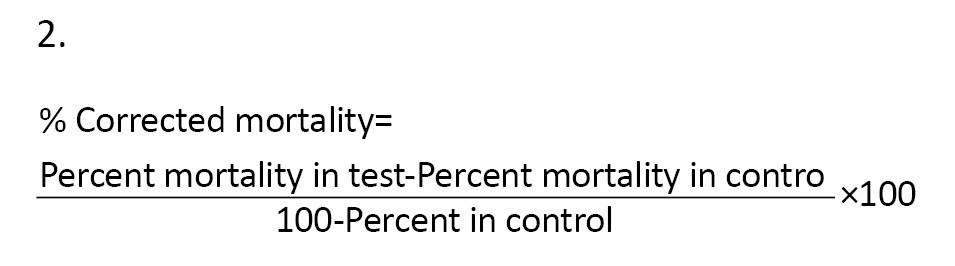
The extract dose was taken as the independent variable, while observed mortalities were the dependent variable. Dimethyl sulfoxide and distilled water were used as negative controls. The temperature and humidity regime at the laboratory was maintained at 28 – 30 °C and 70% - 80%, respectively, and a photoperiod at 12 h light (06.30 – 18.30 hours) alternated with 12 h darkness (18:30 – 06:30 hours).
Statistical analysis
Data on characterization and bioassay on the effect of the crude extracts of U. massaica on larvae of A. gambiae were entered in Excel spreadsheets and appropriately organized for processing. Descriptive statistics were used to determine the quantity of bioactive compounds and the effect of the solvent of extraction, dose, and part of the plant used on exposed larvae. A one-way analysis of variance was used to determine the significance of the impact of extract as a larvicide. All statistical analysis was performed using IBM SPSS statistics software for Windows, version 22 (IBM Corp, Armonk, NY, 2013).
Results
Five major chemical constituents were extracted from U. massaica parts. Those that could not be classified were placed under “other groups.” The order of abundance was phenolic > amino acids and nitrogen derivatives > steroids and terpenoids > flavonoids, nitrogen derivatives, and other groups (Figure 1). Roots produced the highest quantity of constituents [12], followed by leaves [16] and then stems [12]. All the observed abundance, however, did not significantly differ (P<0.05) except for the flavonoids (Table 1).

Individual chemical constituents, however, differed in abundance per chemical group and plant part. Luteolin 6-C-glucoside (flavonoids), neoergosterol and β-sitosterol (steroids and terpenoids), isoleucine (amino acid and nitrogen derivatives), 3,4-diferuloylquinic acid (phenolic compounds) and p-isopropyl aniline (nitrogen derivatives) (Figure 2) were abundant per group and catechin (10.00) in roots, β-sitosterol (7.84) in leaf and 3,4-diferuloylquinic acid (11.43) in stem (Figure 3). Interestingly, extracts from stems were not only more potent than those from leaves or roots, but their effects equaled the WHO threshold of >80% mortality for insecticidal effectiveness for doses of 10 mL/100 cm3 (e/w) and above (Figure 4). Observed mortalities, however, were not significantly different (P>0.05) irrespective of dose or control (Table 2).

Discussion
Different parts of plants (leaves, fruits, seeds, roots, and bark) contain polyphenols or secondary metabolites (flavonols, anthocyanins, and phenolic acids) that are responsible for mosquitocidal properties [35]. Indeed, much of the research on malaria intervention is on the search for novel chemical agents that have equivalent potency as synthetic insecticides but whose activity is specific and friendly to humans and the environment [35, 36]. The study reported herein was one of such, and herein we demonstrate that U. massaica contains several chemical agents that are toxic to A. gambiae larvae. The findings are similar to Justicia adhatoda L. [37] and Acacia nilotica [38] against different mosquito species.
In this study, the roots contained more bioactive compounds than the leaves or stem, though extracts from the stem were more potent than those of the leaves or roots. These findings were similar to those of Baz et al. [39], Anupam et al. [40], and Yugi and Kiplimo [41], who reported on the differential distribution and concentration of bioactive compounds in different plant parts. These findings come when insecticide resistance and environmental challenges [42] posed by synthetic insecticides are a thorn in the flesh against the race to reduce, and if not pacified, malaria infection must be welcomed. Indeed, it adds to the cumulated stock and use of natural products that are not only rich in bioactive compounds but are target-specific and safe for the environment [3, 36]. As such, the findings of this study put extracts of U. massaica among plants that can provide alternative sources of green insecticides [43] for mosquito-borne disease control [33].
The study showed that U. massaica contains secondary metabolites identified as tannins, terpenes, saponins, flavonoids, alkaloids, and phenols. A total of 47 bioactive compounds were extracted in different quantities (roots, 19; leaves, 16; and stem, 12) of the U. massaica plant. This amount was moderate but still higher than recently reported elsewhere [14]. This finding is promising as we believe that with refining, we are likely to realize more compounds. Though the exact nature and the specific bioactive compound responsible for the observed activity were not expressly determined, it is evident from an earlier demonstration that phenolic compounds, flavonoids, and tannins possess insecticidal properties [44] and may have been responsible for the observed toxicity. These findings are comparable with earlier works by Hoesain et al. [45] of plant metabolite potential against Spodoptera litura, Hillary et al. [46] of efficacy of plant products, Bassey et al. [47] of Allium sativum and Murraya koenigii, and Folawewo et al. [48], Oboho et al. [49] of Hippocratea africana with demonstrable mosquitocidal [50] activities. The bioactive compounds’ toxicity was believed to be due to their impairing mitochondrial function in the exposed insect vectors [51].
The present study demonstrates the toxicity of extracts of U. massaica against A. gambiae larvae with activity being dependent on dose, solvent, and part of plant extracted. Though the time of exposure was not tested, the observations reported here agree with those reported by Ubulom et al. [51], Opara et al. [52], and Ghosh et al. [40] on the count of increasing concentration of bioactive compounds in different parts of the plant.
Conclusion
U. massaica bioactive compounds are diverse and have toxicants against A. gambiae larvae. They qualify for exploitation as green insecticides to target and protect against the anthropophilic, endophilic, and endophagic mosquitoes in areas where they are endemic and resist synthetic insecticides.
Study limitations
The study was limited to L3 of laboratory-reared A. gambiae mosquitoes.
Ethical Considerations
Compliance with ethical guidelines
This research was a laboratory-based experimental bioassay. It involved the use of laboratory-sourced and membrane-fed mosquitoes only. There was, therefore, no need for ethical consent.
Funding
The University of Kabianga, Kericho, Kenya funded the project.
Authors contributions
Conceptualization, data analysis, and writing the original draft: Jared Yugi Owiti, supervision, methodology, investigation, data collection, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Richard Amito, Harnel Versey, and Gayle Aurelia for processing and culturing the experimental mosquitoes. Also, the authors appreciate the Centre for Global Health Research (CGHR), Kisumu, for providing laboratory space, experimental mosquitoes, and equipment, respectively.
References
- Parabakan P, Sivasubramanian C, Veeramani R, Prabhu S. Review study on larvicidal and Mosquito Repellent activity of Volatile Oils Isolated from Medicinal Plants. International Journal of Environment, Agriculture and Biotechnology 2017; 2(6):3132-8.[DOI:10.22161/ijeab/2.6.46]
- Bekele D. Review on insecticidal and repellent activity of plant products for malaria mosquito control. Biomedical Research and and Reviews. 2018; 2(2): 1-7. [DOI:10.15761/BRR.1000114]
- Habeeb Rahuman HB, Dhandapani R, Narayanan S, Palanivel V, Paramasivam R, Subbarayalu R, et al. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnology. 2022; 16(4):115-44. [DOI:10.1049/nbt2.12078] [PMID]
- Bhusal KK, Magar SK, Thapa R, Lamsal A, Bhandari S, Maharjan R, et al. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review. Heliyon. 2022; 8(6):e09717. [DOI:10.1016/j.heliyon.2022.e09717] [PMID]
- Amuka O. Efficacy of selected medicinal plants used by the ogiek communities against microbial related infections [PhD dissertation]. Nairobi: Kenyatta University; 2014. [Link]
- Freeman JC, Smith LB, Silva JJ, Fan Y, Sun H, Scott JG. Fitness studies of insecticide resistant strains: Lessons learned and future directions. Pest Management Science. 2021; 77(9):3847-56. [DOI:10.1002/ps.6306] [PMID]
- Chaachouay N, Zidane L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates. 2024; 3(1):184-207. [DOI:10.3390/ddc3010011]
- Nduwamungu J, Ugirabe MA, Mahoro J, Dusingize MC, Kabarungi M, Irimaso E, et al. New insights into the indigenous knowledge of the uses of the common stinging nettle (Urtica massaica Mildbr.) in Rwanda. Cogent Food & Agriculture. 2024; 10(1):2306722. [DOI:10.1080/23311932.2024.2306722]
- Marrassini C, Davicino R, Acevedo C, Anesini C, Gorzalczany S, Ferraro G. Vicenin-2, a Potential anti-inflammatory constituent of Urtica circularis. Journal of Natural Products. 2011; 74(6):1503-7.[DOI:10.1021/np100937e] [PMID]
- Ding XY, Zhang Y, Wang L, Zhuang HF, Chen WY, Wang YH. Collection calendar: The diversity and local knowledge of wild edible plants used by Chenthang Sherpa people to treat seasonal food shortages in Tibet, China. Journal of Ethnobiology and Ethnomedicine. 2021; 17(1):40. [DOI:10.1186/s13002-021-00464-x] [PMID]
- Hassen A. Diversity and potential contribution of wild edible plants to sustainable food security in North Wollo, Ethiopia. Biodiversitas. Journal of Biology and Diversity. 2021; 22(6):2501-10.[DOI:10.13057/biodiv/d220660]
- Kregiel D, Pawlikowska E, Antolak H. Urtica spp.: Ordinary plants with extraordinary properties. Molecules (Basel, Switzerland). 2018; 23(7):1664. [DOI:10.3390/molecules23071664] [PMID]
- Adhikari BM, Bajracharya A, Shrestha AK. Comparison of nutritional properties of stinging nettle (Urtica dioica) flour with wheat and barley flour. Food Science and Nutrition. 2016; 4(1):119-24. [DOI:10.1002/fsn3.259] [PMID]
- Wambui J, Ikedi RIO, Macharia RW, Kama-Kama F, Nyaboga EN. Phytoconstituents of Kenyan stinging nettle (Urtica species) and their molecular docking interactions revealed anti-inflammatory potential as cyclooxygenase-2 inhibitors. Scientific African. 2024; 23:e02088. [DOI:10.1016/j.sciaf.2024.e02088]
- Kamau LN, Mbaabu PM, Karuri PG, Mbaria JM, Kiama SG. Medicinal plants used in the management of diabetes by traditional healers of Narok County, Kenya. Cellmed Orthocellular Medicine and Pharmaceutical Association. 2017; 7(2):e10. [DOI:10.5667/tang.2017.0008]
- Körpe DA, İşerı ÖD, Sahin FI, Cabi E, Haberal M. High-antibacterial activity of Urtica spp. seed extracts on food and plant pathogenic bacteria. International Journal of Food Science and Nutrition. 2013; 64(3):355-62. [DOI:10.3109/09637486.2012.734290] [PMID]
- Kipruto A, Mwamburi L, BII C, Kipngetich B. The antimicrobial activity of the leaves of Urtica massaica on Staphylococcus aureus, Escherichia coli. Journal of Medicinal Plant Studies. 2019; 7(2):21- 4. [Link]
- Shonte TT, De Kock HL. Descriptive sensory evaluation of cooked stinging nettle (Urtica dioica L.) leaves and leaf infusions: Effect of using fresh or oven-dried leaves. South African Journal of Botany. 2017; 110:167-76. [DOI:10.1016/j.sajb.2016.11.010]
- Nsengimana T, Nsengimana V, Nsanganwimana F. Local knowledge and use of wild edible plants in the eastern part of Nyungwe National Park in Rwanda: Prospects for forest biodiversity conservation. Journal of Research in Forestry, Wildlife and Environment 2020; 12(4):66-75. [Link]
- DeFilipps RA, Krupnick GA. The medicinal plants of Myanmar. PhytoKeys. 2018; (102):1-341. [DOI:10.3897/phytokeys.102.24380] [PMID]
- Gahamanyi N, Munyaneza E, Dukuzimana E, Tuyiringire N, Pan CH, Komba EVG. Ethnobotany, ethnopharmacology, and phytochemistry of medicinal plants used for treating human diarrheal cases in Rwanda: A Review. Antibiotics. 2021; 10(10):1231. [DOI:10.3390/antibiotics10101231] [PMID]
- Maniriho O, Nkurunziza JP, Ayodele AE, Benimana F, Murhula HP, Farhan HF, et al. Chemical screening and antimicrobial activities of Rwandan traditional medicinal plant, Urtica massaica Mildbr (Urticaceae). EAS Journal of Pharmacy and Pharmacology. 2021; 3(2):56-63. [DOI:10.36349/easjpp.2021.v03i02.004]
- Şen G, Akbulut S, Karaköse M. Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye). Open Chemistry. 2022; 20(1):873-911. [DOI:10.1515/chem-2022-0204]
- Grauso L, de Falco B, Lanzotti V, Motti R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochemistry Reviews. 2020; 19(6):1341- 77. [DOI:10.1007/s11101-020-09680-x]
- Khatoro RT, Yugi JO, Sudoi V. Ovicidal, Larvicidal and Pupicidal Efficacy of Crude Methanol and Hexane Extract of Urtica massaica Mildbri on Anopheles gambiae Giles. Jordan Journal of Biological Sciences. 2021; 14(3):433 - 40. [DOI:10.54319/jjbs/140308]
- Kothari CR. Research design: Research methodology, methods and techniques. New Delhi: New Age International Publishers; 2004. [Link]
- Sugita P, Amilia R, Arifin B, Utami D, Rahayu C, Dianhar H. The phytochemical screening hexane and methanol extract of Sinyo Nakal (Duranta repens). Asian Journal of Pharmaceutical and Clinical Research 2020; 13(8):196-200. [DOI:10.22159/ajpcr.2020.v13i8.38165]
- Falaki F. Sample preparation techniques for gas chromatography. In: Kusch P, editor. Gas chromatography-derivatization, sample preparation, application. London: IntechOpen. [Link]
- Cheng WL, Markus C, Lim CY, Tan RZ, Sethi SK, Loh TP, et al. Calibration practices in clinical mass spectrometry: Review and recommendations. Annals of Laboratory Medicine. 2023; 43(1):5-18. [DOI:10.3343/alm.2023.43.1.5] [PMID]
- Thomas SN, French D, Jannetto PJ, Rappold BA, Clarke WA. Liquid chromatography- tandem mass spectrometry for clinical diagnostics. Nature Reviews Methods Primers. 2022; 2(1):96. [DOI:10.1038/s43586-022-00175-x] [PMID]
- Vloemans D, Pieters A, Dal Dosso F, Lammertyn J. Revolutionizing sample preparation: A novel autonomous microfluidic platform for serial dilution. Lab on a Chip. 2024; 24(10):2791-801. [DOI:10.1039/d4lc00195h] [PMID]
- WHO. Chemical methods for the control of vectors and pests of public health importance, Geneva, World Health Organization, 1997. Geneva: WHO; 1997. [Link]
- World Health Organization, Global Malaria Programme, Malaria Unit. Indoor residual spraying: Use of indoor residual spraying for scaling up global malaria control and elimination. Geneva: World Health Organization; 2006. [Link]
- Abbot WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925; 18(2):256- 67. [DOI:10.1093/jee/18.2.265a]
- Ramkumar G, Karthi S, Muthusamy R, Natarajan D, Shivakumar MS. Adulticidal and smoke toxicity of Cipadessa baccifera (Roth) plant extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus. Parasitology Reserach. 2015; 114(1):167-73. [DOI:10.1007/s00436-014-4173-5] [PMID]
- Asadollahi A, Khoobdel M, ZahraeiRamazani A, Azarmi S, Mosawi SH. Effectiveness of plantbased repellents against different Anopheles species: A systematic review. Malaria Journal. 2019; 18(1):436. [DOI:10.1186/s12936-019-3064-8] [PMID]
- Thanigaivel A, Senthil-Nathan S, Vasantha-Srinivasan P, Edwin ES, Ponsankar A, Selin-Rani S, et al. Chemicals isolated from Justicia adhatoda Linn reduce fitness of the mosquito, Aedes aegypti L. Archives of Insect Biochemistry and Physiology. 2017; 94(4). [DOI:10.1002/arch.21384] [PMID]
- Vivekanandhan P, Venkatesan R, Ramkumar G, Karthi S, Senthil-Nathan S, Shivakumar MS. Comparative analysis of major mosquito vectors response to seed-derived essential oil and seed pod-derived extract from Acacia nilotica. International Journal of Environmental Research and Public Health. 2018; 15(2):388. [DOI:10.3390/ijerph15020388] [PMID]
- Baz MM, Selim AM, Radwan IT, Alkhaibari AM, Gattan HS, Alruhaili MH, et al. Evaluating larvicidal, ovicidal and growth inhibiting activity of five medicinal plant extracts on Culex pipiens (Diptera: Culicidae), the West Nile virus vector. Scientific Reports. 2024; 14(1):19660. [DOI:10.1038/s41598-024-69449-6] [PMID]
- Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research. 2012; 135(5):581-98. [Link]
- Yugi JO, Kiplimo JJ. Inhibitory effect of crude ethanol and water extracts of Phytolacca dodecandra (L’Herit) on embryonic development of Anopheles gambiae (Diptera: Culicidae). Jordan Journal of Biological Sciences. 2017; 10(3):177 - 83. [Link]
- Govindarajan M, Rajeswary M, Arivoli S, Tennyson S, Benelli G. Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: An eco-friendly tool against malaria, dengue, and lymphatic filariasis mosquito vectors? Parasitology Research. 2016; 115(5):1807-16. [DOI:10.1007/s00436-016-4920-x] [PMID]
- Raimova KV, Abdulladjanova NG, Tashpulatov FN, Juraev SS, Matchanov AD, Rakhimov RN, et al. Comprehensive Study of the Chemical Composition of Urtica dioica L. Journal of Critical Reviews. 2020; 7(5):750-5. [Link]
- Boate UR, Abalis OR. Review on the Bio-insecticidal Properties of Some Plant Secondary Metabolites: Types, formulations, modes of action, advantages and limitations. Asian Journal of Research in Zoology. 2020; 3(4):27-60. [DOI:10.9734/AJRIZ/2020/v3i430099]
- Hoesain M, Suharto, Prastowo S, Pradana AP, Alfarisy FK, Adiwena M. Investigating the plant metabolite potential as botanical insecticides against Spodoptera litura with different application methods, Cogent Food & Agriculture. 2023; 9(1):2229580. [DOI:10.1080/23311932.2023.2229580]
- Hillary VE, Ceasar SA, Ignacimuthu S. Efficacy of plant products in controlling disease vector mosquitoes, a review. Entomologia Experimentalis et Applicata. 2024; 172(3):195-214. [DOI:10.1111/eea.13401]
- Bassey EE, Iduu N, Okonkwo IF, Kyrian-Ogbonna EA. Phytochemical Analysis and In vitro Evaluation of the synergistic Antimicrobial Activity of Allium sativum and Murraya koenigii. International Journal of Applied Science and Engineering. 2014; 4(1):8-16. [Link]
- Folawewo AD, Madu AN, Agbaje-Daniels FV, Faboyede AO, Coker AR. Phytochemical screening and antibacterial activities of the root bark extracts of Hippocratea Africana (Willd.) Loes. ex Engl. European Journal of Medicinal Plants. 2017; 19(1): 1-8. [Link]
- Oboho D, Akpan AU, Aguzie I, Nzewuihe GU. Evaluation of Adulticidal Efficacy of Methanol Leaf Extract of Hippocratea africana WILLD and Lasianthera Africana P. BEAUV against Anopheles gambiae. International Journal of Innovative Science and Research Technology 2020; 5(6):50-57. [Link]
- Guo C, Wang L, Chen N, Zhang M, Jia J, Lv L, et al. Advances in research and utilization of botanical pesticides for agricultural pest management in Inner Mongolia, China. Chinese Herbal Medicines. 2023; 16(2):248-62. [DOI:10.1016/j.chmed.2023.04.002] [PMID]
- Edwin UP, Nyiutaha IG, Essien AE, Nnamdi OK, Sunday EM. Larvicidal effect of aqueous and ethanolic extracts of Senna alata on Anopheles gambiae, Culex quinquefasciatus and Aedes aegypti. Pakistan Journal of Pharmaceutical Science. 2013; 26(3):561-6. [PMID]
- Opara KN, Udoidung NI, Ubulom PME, Chikezie FM. Ononiwu NE. Larvicidal Activity of Sacoglottis gabonnensis stem bark extracts against Culex quinquef asciatus and Aedes aegypti mosquitoes in Uyo, Nigeria. International Journal of Tropical Disease and Health. 2017; 24(1):1-6. [DOI:10.9734/IJTDH/2017/33710]
Type of Study: Original Article |
Subject:
Health
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |